

Development of Protein Separation Membranes and Their Progress
† These authors contributed equally to this work.
Received date: 2024-03-08
Revised date: 2024-05-27
Online published: 2025-03-28
Supported by
National Natural Science Foundation of China(21476249)
Natural Science Foundation of Tianjin(18JCZDJC37100)
Membrane separation technology has been intensively used in numerous applications such as seawater desalination, water treatment and reuse, fine separation and product concentration, biomedical treatment and so forth owing to its low operation temperature, easy operation process, modularity, and high separation efficiency. However, due to membrane materials, membrane structures, and membrane manufacturing technology, the trade-off behavior between the water flux and the rejection rate of conventional separation membranes has become a technical bottleneck. The preparation of high-performance separation membranes using proteins as membrane materials is expected to break the trade-off behavior of conventional separation membranes. Protein separation membrane works super-efficiently for the target separation and transport, as well as the antibacterial and antifouling properties, where an emerging membrane material of proteins can transport the solute due to their inherent specific water or ion channels, rich binding sites with metal ions, regular nanostructures or low-cost and multifunctional. In this review, the widely implemented membrane materials and fabrication strategies for protein separation membranes are summarized in detail, and the research progress of the various protein separation membranes is described. Furthermore, the challenges faced by protein separation membranes are comprehensively reviewed. This review provides some insights into the construction and prospect of protein separation membranes.
1 Introduction
2 Novel protein materials for membrane fabrication
2.1 Protein containing channels
2.2 Protein containing rich binding sites with metal ions
2.3 Protein containing regular nanostructures
2.4 Low-cost and multifunctional protein
3 Fabrication methods of protein separation membranes
3.1 Amyloid-like assembly
3.2 Interfacial polymerization
3.3 Layer-by-layer self-assembly
3.4 Mussel-inspired biomimetic co-deposition
3.5 Other methods
4 Research progress of protein separation membranes
4.1 Aquaporin biomimetic separation membranes
4.2 Lysozyme separation membranes
4.3 Protein separation membrane for chelating metal ions
4.4 Other protein separation membrane
5 Conclusion and outlook
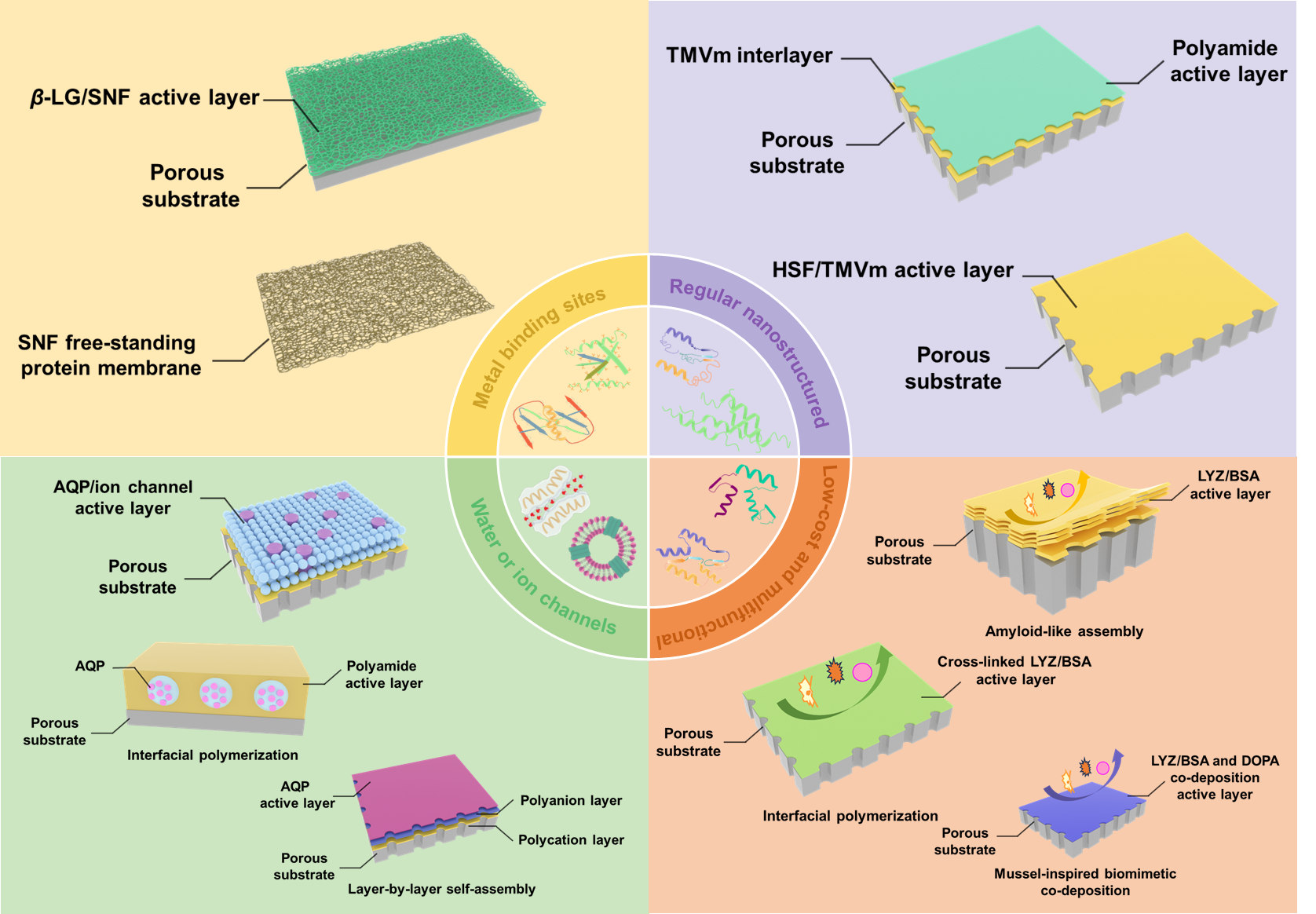
Key words: protein; protein separation membrane; permeability; selectivity; construction
Junping Miao , Zhaoqian Zhang , Shaopeng Xin , Yunxia Hu . Development of Protein Separation Membranes and Their Progress[J]. Progress in Chemistry, 2025 , 37(2) : 195 -210 . DOI: 10.7536/PC240312
表1 氨基酸中常见官能团与金属离子之间的结合常数K[41⇓⇓⇓⇓-46]Table 1 The binding constant K between functional groups and metal ions[41⇓⇓⇓⇓-46] |
| Ions | -OH | -NH2 | -COOH | -SH | Imidazolyl |
|---|---|---|---|---|---|
| Mn2+ Co2+ Ni2+ Cu2+ Zn2+ Cd2+ Pb2+ | 4.35 6.08 9.72 9.72 8.77 2.47 10.12 | 4.66 5.12 5.45 4.42 5.39 4.90 3.51 | 1.56 10.89 0.89 4.45 11.93 6.65 14.65 | 3.20 9.04 5.00 2.43 3.94 5.53 7.98 | 3.10 1.66 0.78 0.85 5.50 1.10 1.48 |
图5 (a)以蛋白为水相单体[117]、(b)以具有规则纳米结构的蛋白构建中间层[118]和(c)以含功能蛋白的囊泡为水相添加剂[26],通过IP法制备蛋白分离膜的示意图Fig. 5 Schematic illustration of the fabrication process of the protein separation membranes via IP using protein as (a) an aqueous monomer [117], (b) an interlayer [118], and (c) an aqueous additive [26]. Copyright 2017, Elsevier; Copyright 2020, American Chemical Society; Copyright 2015, Elsevier |
图6 AQP囊泡印迹膜的制备过程示意图[133]。(1)AQP囊泡,(2)多孔醋酸纤维素膜基底,(3)固定在多孔膜上的AQP囊泡,(4)AQP囊泡印迹膜和(5)AQP囊泡印迹膜的断面结构Fig. 6 The schematic fabrication process of the AQP-vesicle-imprinted membrane[133]. (1) vesicles containing AQP, (2) porous cellulose acetate membrane substrate, (3) vesicles containing AQP immobilized on the porous membrane e, (4) AQP-vesicle-imprinted membrane, and (5) cross-section of the AQP-vesicle-imprinted membrane. Copyright 2013, The Royal Society of Chemistry |
图7 (a)含AQP囊泡通过吸附、破裂融合后铺展到基膜表面制备AQP仿生膜;(b)封装含AQP囊泡到基膜表面制备AQP仿生膜[10]Fig. 7 Schematic diagram of preparing AQP biomimetic membranes by (a) adsorbing, rupturing, fusing, and then spreading AQP containing vesicles onto the surface of the substrate or (b) encapsulating vesicles containing AQP onto the surface of the substrate[10]. Copyright 2015, Elsevier |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
(林立刚, 郑甜甜, 强荣荣. 纺织导报, 2018(S1): 99.).
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
(顾正阳, 龚超, 杨望臻, 于水利, 姚启翰. 化工进展, 2018, 37(3): 1037.
|
| [14] |
(曾艳军, 张林, 陈欢林. 中国工程科学, 2014, 16(7): 10.).
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
(李俊敏, 刘朝晖, 尚忠林. 河北师范大学学报, 2005, 29(5): 519.).
|
| [30] |
|
| [31] |
|
| [32] |
(王克威. 科学, 2006, 4: 13.)
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
(孙越宜, 唐科梦, 杨明英, 朱良均. 蚕桑通报, 2016, 47(2): 26.)
|
| [49] |
|
| [50] |
(郝明浩. 山东师范大学硕士论文, 2019.).
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
(吴晨星. 苏州大学硕士论文, 2020.).
|
| [58] |
|
| [59] |
(陈婷. 西南石油大学硕士论文, 2015).
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
(曹阳, 任玉灵, 郭世伟, 万印华, 罗建泉. 化工进展, 2020, 39(6): 2125.)
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
(杨秀丽. 河北工业大学硕士论文, 2015).
|
| [121] |
(何帅, 梁靖媚, 胡惠兰, 袁嘉男, 何剑波, 倪照飞. 材料导报, 2016, 30(6): 1.).
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
(胡云霞, 安红匣, 朱寒超, 龚耿浩. EP 115920649A, 2021.).
|
| [126] |
(田欣欣, 王暄, 彭维, 吕晓龙, 于越, 袁晓彤. 膜科学与技术, 2021, 41(1): 152.)
|
| [127] |
|
| [128] |
|
| [129] |
(安红匣. 天津工业大学硕士论文, 2023.).
|
| [130] |
(朱寒超. Master Dissertation of Tiangong University, 2022.).
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
/
| 〈 |
|
〉 |