

Non-Fullerene Acceptor Materials: Selenophenes Engineering
Received date: 2024-05-08
Revised date: 2024-10-31
Online published: 2025-03-28
Supported by
Department of Science and Technology of Inner Mongolia, China(2020GG0192)
Natural Science Foundation of Inner Mongolia, China(2022ZD04)
Inner Mongolia Normal University, China(112/1004031962)
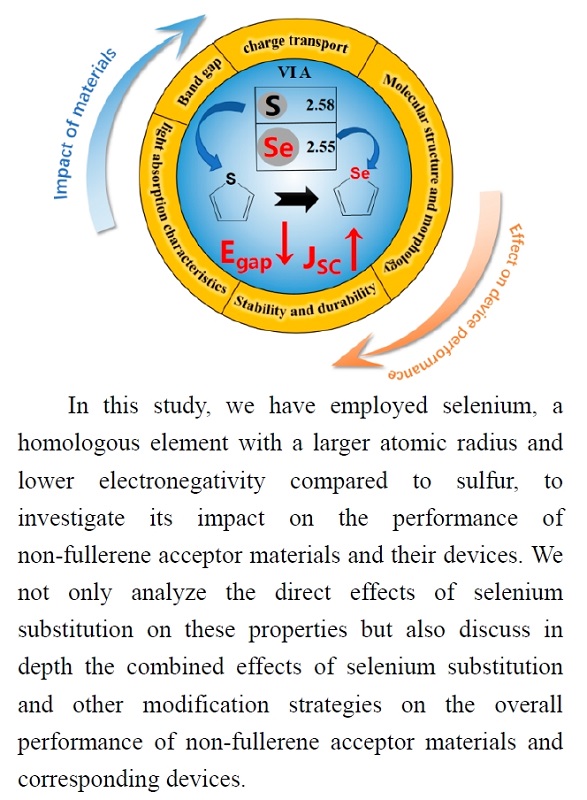
To advance the development of high-performance organic solar cells, in recent years, the academic community has conducted in-depth research on the design of non-fullerene acceptor materials and the interplay between their structure and properties. Structural modifications of these materials involve optimization of the core structure, side chain engineering, expansion of the conjugated system, and doping with heteroatoms. Focusing on sulfur, due to its outstanding semiconducting properties, it is widely used in the manufacturing of electronic materials and semiconductor devices, especially in the field of organic solar cells. Selenium, as a homologous element of sulfur, not only shares similar chemical and physical properties but also possesses unique characteristics. For instance, compared to sulfur, selenium has a larger atomic radius, which provides additional space within molecules, facilitating charge transfer and improving electron distribution. Moreover, due to its greater mass, selenium atoms have lower vibrational frequencies, a characteristic that enhances light absorption capabilities within the visible spectrum. Therefore, the introduction of selenium atoms is considered a potential approach to enhancing the efficiency of organic solar cells. This review focuses on the impact of the position and ratio of selenium atoms in condensed-ring electron acceptors (such as ITIC and Y6 derivatives) and certain non-condensed ring acceptors on their photovoltaic performance. It also discusses the synergistic effect of selenium atom substitution with other optimization strategies and its comprehensive impact on the performance of various types of organic solar cells (including small molecule, polymer, and all-polymer solar cells).
1 Introduction
2 Research on the Regulation of Photovoltaic Performance by Selenophenes for Non-Fullerene Electron Acceptors with Condensed Rings
2.1 Research on the regulation of photovoltaic performance by selenophenes for ITIC series acceptor materials
2.2 Research on the regulation of photovoltaic performance by selenophenes for Y series acceptor materials
2.3 Research on the regulation of photovoltaic performance by selenophenes for Y series polymer materials
2.4 Research on the regulation of photovoltaic performance by selenophenes for other types of acceptor materials
3 Research on the Regulation of photovoltaic Performance by Selenophenes for Non-Condensed Ring Non-Fullerene Electron Acceptors
4 Conclusion and outlook
Key words: organic solar cells; selenophenes; narrow bandgap; broad absorption
Huan Liu , Huimin Shi , Haiyang Li , Chuanlang Zhan . Non-Fullerene Acceptor Materials: Selenophenes Engineering[J]. Progress in Chemistry, 2025 , 37(2) : 235 -254 . DOI: 10.7536/PC240416
图2 含硒吩的ITIC系列衍生物受体化学结构Fig. 2 Chemical structure of ITIC series derivatives containing selenophene |
表1 含硒吩的ITIC系列衍生物受体中的光学、电化学和光伏性能Table 1 Optical, electrochemical and photovoltaic properties of ITIC series derivatives containing selenium atom |
| Donor | Acceptor | EHOMO(eV) | ELUMO(eV) | VOC(V) | JSC(mA/cm2) | FF | PCE(%) | Refs. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| PM6 | C8T-BDT4Cl | -5.75 | -3.92 | 0.86 | 19.65 | 72.32 | 12.21 | 14 | ||
| PM6 | C8T-BDSe4Cl | -5.71 | -3.96 | 0.85 | 21.79 | 72.71 | 13.50 | 14 | ||
| J51 | IDTIDSe-T-IC | -5.41 | -3.81 | 0.91 | 15.16 | 58.0 | 7.84 | 14 | ||
| J71 | SeCT-IC | -5.53 | -3.92 | 0.889 | 18.09 | 67.69 | 10.89 | 16 | ||
| J71 | CSeT-IC | -5.60 | -3.91 | 0.912 | 17.17 | 54.83 | 8.52 | 16 | ||
| J71 | CTSe-IC | -5.55 | -3.90 | 0.925 | 18.21 | 68.81 | 11.59 | 16 | ||
| PBDB-T-2F | SRID-4F | -5.52 | -3.90 | 0.846 | 20.21 | 75.2 | 13.05 | 17 | ||
| PBDB-T-2F | TRID-4F | -5.52 | -3.90 | 0.890 | 18.45 | 75.0 | 12.33 | 17 | ||
| PBDB-T | SeIN | -5.71 | -4.13 | 0.88 | 17.4 | 56.0 | 8.61 | 18 | ||
| PBDB-T | SeOut | -5.72 | -4.17 | 0.88 | 16.9 | 62.0 | 9.25 | 18 | ||
| PBDB-T | SeInMe | -5.7 | -4.11 | 0.91 | 15.9 | 61.0 | 8.80 | 18 | ||
| PBDB-T | SeOutMe | -5.7 | -4.13 | 0.9 | 16.7 | 64.0 | 9.68 | 18 | ||
| PM6 | SeTIC | -5.55 | -3.90 | 0.95 | 15.45 | 51.0 | 7.46 | 19 | ||
| PM6 | SeTIC4Cl | -5.65 | -4.08 | 0.78 | 22.92 | 75.0 | 13.32 | 19 | ||
| PBT1-C | TSeIC-M-Cl | -5.64 | -4.01 | 0.82 | 16.10 | 73.60 | 9.72 | 20 | ||
| PBT1-C | TSeIC-P-Cl | -5.62 | -3.98 | 0.85 | 18.67 | 71.00 | 11.26 | 20 | ||
| PM6 | BDSeIC | -5.53 | -3.92 | 0.97 | 14.00 | 51.9 | 7.10 | 21 | ||
| PM6 | BDSeIC2Br | -5.63 | -3.99 | 0.89 | 20.3 | 69.1 | 12.5 | 21 | ||
| PM6 | BDSeIC4Br | -5.65 | -4.02 | 0.85 | 16.40 | 68.6 | 9.60 | 21 | ||
| PM6 | TSeIC4Cl | -5.75 | -3.99 | 0.74 | 20.92 | 71.70 | 11.13 | 22 | ||
| PM6 | TSeIC4Br | -5.69 | -3.97 | 0.77 | 21.27 | 72.40 | 11.92 | 22 | ||
| PM7 | BDSe-4Cl | -5.63 | -3.94 | 0.83 | 22.50 | 74.1 | 13.83 | 23 | ||
| PM7 | BDSe-2(BrCl) | -5.64 | -3.95 | 0.83 | 22.91 | 76.5 | 14.54 | 23 | ||
| PM7 | BDSe-4Br | -5.65 | -3.96 | 0.83 | 22.12 | 71.5 | 13.12 | 23 | ||
| PM7 | BDSeThCl | -5.58 | -3.82 | 0.97 | 17.85 | 68.8 | 11.91 | 24 | ||
| PM7 | BDSePhCl | -5.61 | -3.96 | 0.92 | 20.35 | 73.1 | 13.68 | 24 | ||
| PM6 | TTTIC | -5.69 | -3.90 | 0.94 | 18.29 | 70.10 | 12.05 | 25 | ||
| PM6 | TSeTIC | -5.65 | -3.91 | 0.93 | 19.42 | 75.90 | 13.71 | 25 | ||
| PBDB-T | IDT2Se | -5.41 | -3.87 | 0.89 | 17.49 | 60.8 | 9.36 | 26 | ||
| PBDB-T | IDT2Se-4F | -5.51 | -4.0 | 0.79 | 21.49 | 65.9 | 11.19 | 26 | ||
| PBDB-T | IDTO-Se-4F | -5.49 | -3.90 | 0.831 | 18.55 | 69.2 | 10.67 | 27 | ||
| PBDB-T | IDT2Se-C2C4-F | -5.36 | -5.43 | 0.81 | 19.19 | 65.6 | 10.2 | 28 | ||
| PBDB-T | IDT2Se-C2C4-F | -5.43 | -3.5 | 0.77 | 22.02 | 62.3 | 10.57 | 28 | ||
| PM6 | MQ3 | -5.63 | -3.94 | 0.91 | 22.19 | 66.92 | 13.51 | 29 | ||
| PM6 | MQ5 | -5.59 | -3.95 | 0.86 | 24.48 | 74.31 | 15.64 | 29 | ||
| PM6 | MQ6 | -5.61 | -3.95 | 0.88 | 24.62 | 75.66 | 16.39 | 29 | ||
| PBT1-C | SePT-IN | -5.80 | -3.97 | 0.878 | 13.92 | 72.9 | 8.91 | 30 | ||
| PM6 | T-Se | -5.61 | -3.83 | 0.953 | 12.80 | 62.1 | 7.44 | 31 | ||
| PM6 | T-Se-4F | -5.62 | -3.93 | 0.781 | 18.09 | 66.6 | 9.41 | 31 | ||
| PM6 | T-Se-Ph | -5.61 | -3.88 | 0.912 | 16.90 | 66.8 | 10.29 | 31 | ||
| PBDB-T | DTPPSe-IC | -5.38 | -3.84 | 0.90 | 17.32 | 66.36 | 9.88 | 32 | ||
| PBDB-T | DTPPSe-2F | -5.52 | -4.05 | 0.84 | 22.16 | 73.70 | 13.76 | 32 | ||
| PBDB-T | DTPPSe-4F | -5.53 | -4.10 | 0.78 | 21.18 | 72.84 | 12.03 | 32 | ||
| PBT1-C | SePTT-2F | -5.71 | -4.00 | 0.830 | 17.51 | 75.0 | 10.90 | 33 | ||
| PBT1-C | SePTTT-2F | -5.66 | -3.97 | 0.895 | 18.02 | 75.9 | 12.24 | 33 | ||
图3 含硒吩的Y系列衍生物受体化学结构Fig. 3 Chemical structure of Y-series derivatives containing selenophene |
表2 含硒吩的Y系列衍生物受体光学、电化学和光伏性能Table 2 Optical, electrochemical and photovoltaic properties of Y-series derivatives containing selenophene |
| Donor | Acceptor | EHOMO(eV) | ELUMO(eV) | VOC(V) | JSC(mA/cm2) | FF | PCE(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| D18 | Y6-Se | -5.72 | -4.11 | 0.839 | 27.98 | 75.3 | 17.5 | 34 |
| PM6 | Se46 | -5.62 | -3.89 | 0.879 | 26.22 | 80.1 | 18.46 | 35 |
| PM6 | BTSeIC-2Cl-γ | -5.48 | -3.68 | 0.86 | 23.72 | 73.08 | 14.91 | 36 |
| PM6 | BTSeIC-2Cl-mix | -5.47 | -3.70 | 0.85 | 23.20 | 71.91 | 14.21 | 36 |
| PM6 | BTSeIC-4Cl | -5.51 | -3.73 | 0.82 | 26.92 | 73.11 | 16.14 | 36 |
| PM6 | Y6-Se-4Cl(120 nm) | -5.73 | -4.42 | 0.84 | 25.9 | 67.2 | 15.0 | 37 |
| PM6 | Y6-Se-4Cl(100 nm): Y6(20 nm) | - | - | 0.84 | 26.3 | 73.0 | 16.4 | 37 |
| PM6 | Y6-Se-4Cl(100 nm): IT-4Cl(20 nm) | - | - | 0.83 | 27.3 | 68.5 | 15.8 | 37 |
| SZ5 | BPS-4F | -5.54 | -4.00 | 0.822 | 25.3 | 77.3 | 16.1 | 38 |
| PM6 | CH1007 | -5.59 | -3.97 | 0.820 | 27.03 | 72.00 | 15.95 | 39 |
| PM6 | L8-BO:T9SBO-F | - | - | 0.881 | 27.1 | 79.6 | 19.0 | 40 |
| PM6 | CH1007 | -5.65 | -388 | 0.825 | 27.50 | 77.0 | 17.47 | 41 |
| PM6 | Se-EH | -5.60 | -3.84 | 0.859 | 27.35 | 79.1 | 18.58 | 41 |
| PM6 | Se-EHp | -5.64 | -3.87 | 0.847 | 27.79 | 76.3 | 17.96 | 41 |
| PM6 | Se-EH:CH1007 | - | - | 0.847 | 28.19 | 79.7 | 19.03 | 41 |
| PM6 | mBzS-4F | -5.60 | -3.90 | 0.804 | 27.72 | 76.35 | 17.02 | 42 |
| PM6 | EHBzS-4F | -5.61 | -3.86 | 0.825 | 27.58 | 70.07 | 15.94 | 42 |
| PM6 | PN6SBO-4F | -5.63 | -3.87 | 0.825 | 23.13 | 66.65 | 12.73 | 42 |
| PM6 | AN6SBO-4F | -5.60 | -3.88 | 0.822 | 15.20 | 62.98 | 8.32 | 42 |
| PM6 | EHN6SEH-4F | -5.59 | -3.89 | 0.809 | 27.80 | 74.64 | 17.48 | 42 |
| PM6 | Y-SeNF | -5.68 | -4.03 | 0.839 | 27.55 | 75.90 | 17.54 | 43 |
| PM6 | L8-BO:Y-SeNF | - | - | 0.873 | 27.88 | 79.20 | 19.28 | 43 |
| PM6 | BTPSeV-4F | -5.35 | -4.00 | 0.66 | 30.1 | 71.4 | 14.2 | 44 |
| PM6 | mPh4F-TT | -5.63 | -3.87 | 0.864 | 25.32 | 77.0 | 16.88 | 45 |
| PM6 | mPh4F-ST | -5.64 | -3.92 | 0.832 | 26.71 | 78.0 | 17.57 | 45 |
| PM6 | mPh4F-TS | -5.61 | -3.92 | 0.825 | 27.59 | 79.0 | 18.05 | 45 |
| PM6 | Y6-Se | -5.55 | -3.83 | 0.82 | 25.47 | 75.0 | 16.02 | 46 |
| PM6 | Y6-2Se | -5.58 | -3.84 | 0.83 | 24.32 | 70.0 | 14.94 | 46 |
| PM6 | A-WSSe-Cl | -5.7 | -3.86 | 0.85 | 26.58 | 77.5 | 17.51 | 47 |
| PM6 | S-WSeSe-Cl | -5.66 | -3.88 | 0.828 | 26.35 | 73.41 | 16.01 | 47 |
| PM1 | S-CSeF | -5.65 | -3.90 | 0.850 | 26.5 | 72.2 | 16.3 | 48 |
| PM1 | A-/SeF | -5.65 | -3.88 | 0.885 | 26.6 | 74.6 | 17.6 | 48 |
| PM1 | A-OSeF | -5.66 | -3.89 | 0.880 | 27.2 | 77.3 | 18.5 | 48 |
| PM6 | AYT11Se9-Cl | -5.69 | -3.89 | 0.842 | 26.86 | 77.47 | 17.52 | 49 |
| PM6 | AYT9Se11-Cl | -5.68 | -3.88 | 0.843 | 27.40 | 78.43 | 18.12 | 49 |
| PM6 | BTPOSe-4F | -5.75 | -3.81 | 0.886 | 24.79 | 76.5 | 16.8 | 50 |
| PM6 | BTPOSe-4F:PC71BM | - | - | 0.89 | 25.42 | 76.6 | 17.33 | 50 |
| D18 | AsymSSe-2F | -5.59 | -4.11 | 0.84 | 27.44 | 79.46 | 18.31 | 51 |
| D18 | AsymSSe-2Cl | -5.58 | -4.13 | 0.841 | 27.56 | 76.24 | 17.68 | 51 |
| D18 | S9TBO-F | -5.61 | -3.84 | 0.838 | 28.19 | 74.93 | 17.71 | 52 |
| D18 | BS3TSe-4F | -5.60 | -3.86 | 0.828 | 29.40 | 75.94 | 18.48 | 52 |
| D18 | BS3TSe-4F:Y6-O | - | - | 0.845 | 29.41 | 76.56 | 19.03 | 52 |
| MPhS-2 | SSe-NIC | -5.53 | -4.02 | 0.876 | 26.29 | 73.08 | 16.83 | 53 |
| PM6 | PzIC-SS-4F | -5.68 | -3.81 | 0.886 | 27.30 | 70.40 | 17.02 | 54 |
| PM6 | PzIC-SSe-4F | -5.63 | -3.83 | 0.872 | 27.44 | 73.5 | 17.58 | 54 |
| PM6 | PzIC-SeSe-4F | -5.62 | -3.83 | 0.873 | 27.7 | 73.12 | 17.69 | 54 |
图4 含硒吩的Y系列聚合衍生物化学结构Fig. 4 Chemical structure of Y-series polymer derivatives containing selenophe |
表3 含硒吩的Y系列聚合衍生物光学、电化学和光伏性能Table 3 Optical, electrochemical and photovoltaic properties of Y-series polymer derivatives containing selenophene |
| Donor | Acceptor | EHOMO (eV) | ELUMO(eV) | VOC(V) | JSC(mA/cm2) | FF | PCE(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| D18 | PYSe-2FT | -5.66 | -3.85 | 0.916 | 22.79 | 64.98 | 13.56 | 58 |
| PM6 | PY2Se-F | -5.76 | -3.93 | 0.885 | 24.4 | 72.2 | 15.6 | 59 |
| PM6 | PY2Se-Cl | -5.75 | -3.93 | 0.884 | 24.5 | 74.3 | 16.1 | 59 |
| PM6 | PY2Se-Cl-o | -5.75 | -3.93 | 0.888 | 24.48 | 74.3 | 16.17 | 60 |
| PM6 | PY2Se-Cl-m | -5.70 | -3.87 | 0.955 | 5.22 | 35.3 | 17.6 | 60 |
| PM6 | PY2Se-Cl-ran | -5.73 | -3.91 | 0.903 | 24.16 | 74.4 | 16.23 | 60 |
| PM6 | PYT-1S1Se | -5.61 | -3.75 | 0.926 | 24.1 | 73.0 | 16.3 | 61 |
| PM6 | PYT-2Se | -5.59 | -3.79 | 0.908 | 23.9 | 71.4 | 15.5 | 61 |
| PM6 | PY-1S1Se | -5.65 | -3.80 | 0.927 | 24.62 | 72.30 | 16.5 | 62 |
| PM6 | PY-1S1Se:PY-2Cl | - | - | 0.914 | 25.74 | 77.2 | 18.2 | 62 |
| PM6 | PY-1S1Se:PY-2Cl | - | - | 0.910 | 25.51 | 76.7 | 17.8 | 62 |
| PM6 | PYT-1S1Se | -5.61 | -3.62 | 0.928 | 24.64 | 73.72 | 16.86 | 63 |
| PM6 | PYT-1S1Se:PYT-1S1Se-4Cl | - | - | 0.902 | 25.50 | 77.12 | 17.74 | 63 |
| PM6 | PYT-1S1Se-4Cl | -5.67 | -3.64 | 0.874 | 24.62 | 73.57 | 15.83 | 63 |
| PBDB-T | PY-Se | -5.56 | -3.77 | 0.891 | 23.52 | 73.85 | 15.48 | 64 |
| PBDB-T | PS-Se | -5.63 | -3.85 | 0.874 | 23.27 | 68.0 | 13.83 | 65 |
| PBDB-T | PN-Se | -5.69 | -3.88 | 0.907 | 24.82 | 71.8 | 16.16 | 65 |
| PBDB-T | PFY-0Se | -5.68 | -3.88 | 0.904 | 20.9 | 68.8 | 13.0 | 66 |
| PBDB-T | PFY-1Se | -5.68 | -3.89 | 0.894 | 21.2 | 72.9 | 13.8 | 66 |
| PBDB-T | PFY-2Se | -5.64 | -3.91 | 0.875 | 23.4 | 72.0 | 14.7 | 66 |
| PBDB-T | PFY-3Se | -5.65 | -3.92 | 0.871 | 23.6 | 73.7 | 15.1 | 66 |
| PBDB-T | PY3Se-1V | -5.51 | -3.90 | 0.81 | 24.7 | 65.9 | 13.2 | 67 |
| PM6 | DIBP3F-S | -6.03 | -4.29 | 0.901 | 24.86 | 72.0 | 16.11 | 68 |
| PM6 | DIBP3F-Se | -6.00 | -4.27 | 0.917 | 25.92 | 76.1 | 18.09 | 68 |
| PM6 | Y-Se | -5.71 | -3.85 | 0.823 | 28.03 | 74.2 | 17.08 | 69 |
| PM6 | DYSe-1 | -5.67 | -3.82 | 0.885 | 27.51 | 76.6 | 18.56 | 69 |
| PM6 | DYSe-2 | -5.68 | -3.83 | 0.884 | 27.45 | 75.2 | 18.22 | 69 |
图5 含硒吩的其他类型受体化学结构Fig. 5 Chemical structure of other types of receptors containing selenophene |
表4 含硒吩的其他类型受体光学、电化学和光伏性能Table 4 Optical, electrochemical and photovoltaic properties of other types of receptors containing selenophene |
| Donor | Acceptor | EHOMO(eV) | ELUMO(eV) | VOC(V) | JSC(mA/cm2) | FF | PCE(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| SM1 | MPU4 | -5.65 | -3.96 | 0.99 | 14.91 | 64.0 | 8.96 | 70 |
| SM1 | PC71BM, MPU4 | - | - | 0.92 | 16.32 | 67.0 | 10.05 | 70 |
| PCDTBT | MPU2 | -5.72 | -4.15 | 0.85 | 14.73 | 64.2 | 8.04 | 71 |
| PCDTBT | MPU5 | -5.75 | -4.00 | 0.94 | 16.32 | 66.0 | 10.12 | 71 |
| TDTBTA | MPU6 | -5.54 | -3.7 | 1.04 | 20.38 | 62.0 | 13.14 | 72 |
| SM1 | PC71BM:MPU6 | - | - | 0.97 | 22.65 | 71.0 | 15.6 | 72 |
| P | MPU7 | -5.54 | -4.02 | 0.88 | 23.25 | 69.0 | 14.12 | 73 |
图6 含硒吩的非稠环受体化学结构Fig. 6 Chemical structure of non-fused ring receptors containing selenophene |
表5 含硒吩的非稠环受体光学、电化学和光伏性能Table 5 Optical, electrochemical and photovoltaic properties of non-fused ring receptors containing selenophene |
| Donor | Acceptor | HOMO(eV) | LUMO(eV) | VOC(V) | JSC(mA/cm2) | FF | PCE(%) | Ref |
|---|---|---|---|---|---|---|---|---|
| PM6 | 2T2Se-F | -5.52 | -3.83 | 0.875 | 20.63 | 66.9 | 12.17 | 77 |
| D18 | TT-O-2F | -5.62 | -3.85 | 0.90 | 19.66 | 58.85 | 10.43 | 78 |
| D18 | TT-S-2F | -5.59 | -3.60 | 0.83 | 24.23 | 76.10 | 15.29 | 78 |
| D18 | TT-Se-2F | -5.61 | -3.63 | 0.76 | 23.82 | 67.50 | 12.23 | 78 |
| PBDB-TF | A4T-16 | -5.67 | -3.97 | 0.889 | 20.20 | 75.7 | 13.60 | 79 |
| PBDB-TF | ASe-1 | -5.67 | -3.98 | 0.835 | 20.70 | 73.8 | 12.70 | 79 |
| PBDB-TF | ASe-2 | -5.65 | -3.98 | 0.844 | 18.60 | 70.1 | 11.00 | 79 |
| PBDB-TF | ASe-3 | -5.61 | -4.14 | 0.789 | 19.70 | 66.7 | 10.40 | 79 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
徐翔, 李坤, 魏擎亚, 袁俊, 邹应萍. 化学进展, 2021, 33(2): 165.
(
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
(薛朝鲁门, 刘宛茹, 白图雅, 韩明梅, 莎仁, 詹传郎. 化学进展, 2022, 34(2): 447.)
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
/
| 〈 |
|
〉 |