

Research Progress for Design and Fabrication of Fluorine-Free Surfaces with Oil-Repellent Property
Received date: 2024-05-08
Revised date: 2024-11-09
Online published: 2025-02-07
Supported by
National Natural Science Foundation of China(22372087)
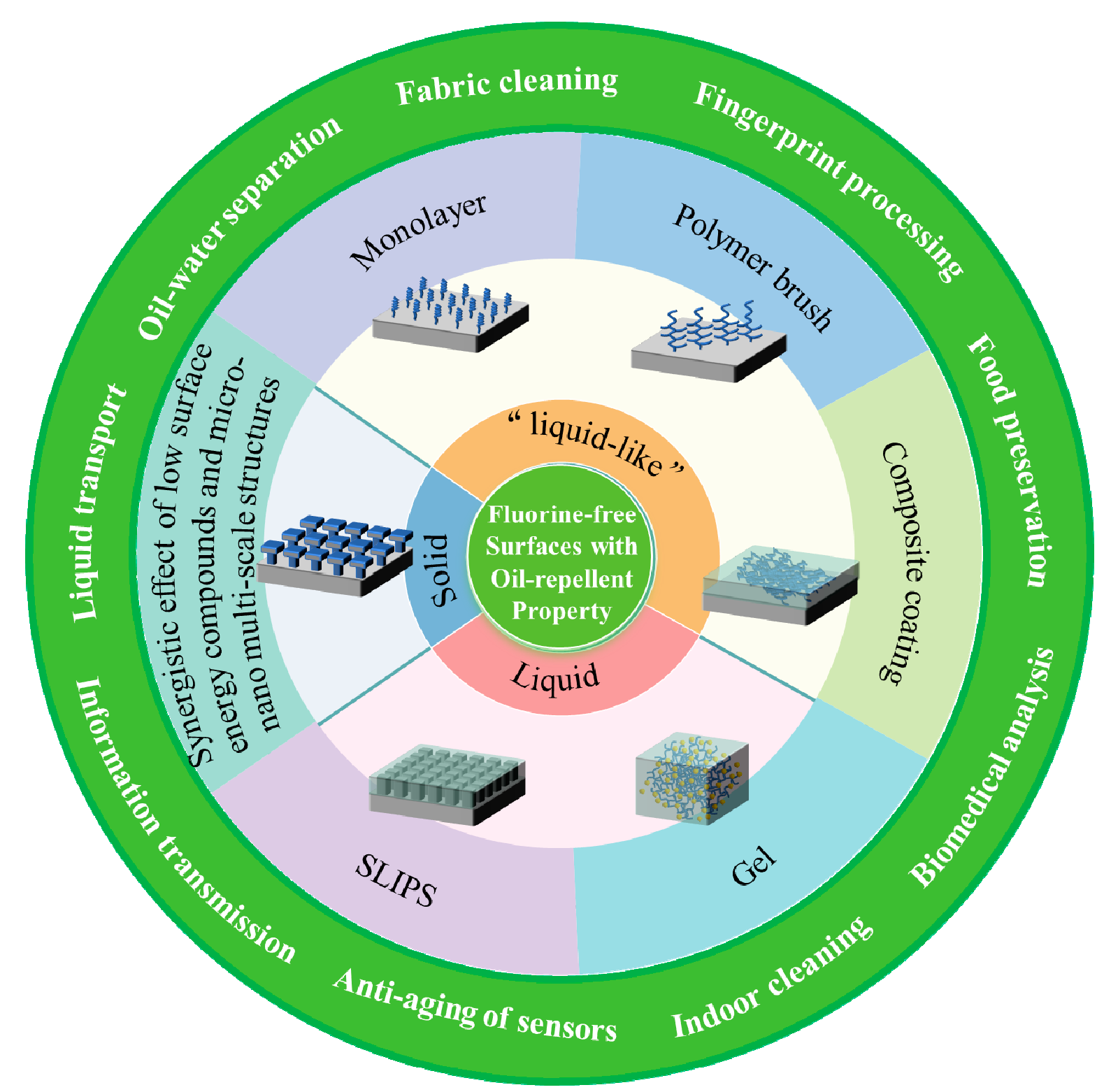
The exceptional waterproof and oil-repellent properties of fluorides, attributed to their remarkably low surface energy, have rendered them extensively employed in the realm of functional finishing. However, the use of fluorine presents potential hazards to human health and engenders irreversible harm to the environment. Consequently, it is progressively being regulated by nations, and discovering alternatives without fluorine has emerged as an imperative concern that necessitates immediate attention in the fields of waterproofing and anti-fouling. To clarify the definition of the fluorine-free materials with oil-repellent property and explore their potential applications in the field of chemistry, the research background of fluorine-free surfaces with oil-repellent property was described, along with a comprehensive review and evaluation of recent achievements and preparation methods. Furthermore, the mechanism of fluorine-free surfaces with oil-repellent property was analyzed, and the application status of fluorine-free coating with oil-repellent property in textiles, construction, food, liquid treatment and other fields was summarized. Additionally, an analysis of the current challenges in ongoing research process of fluorine-free surfaces with oil-repellent property was provided. Finally, a prospective outlook on the future of green and environmentally-friendly fluorine-free surface technology was prospected.
1 Introduction
2 Properties and characteristics of fluorine-free surfaces with oil-repellent property
3 Preparation strategy of fluorine-free surfaces with oil-repellent property
3.1 Solid fluorine-free surfaces with oil-repellent property
3.2 Liquid fluorine-free surfaces with oil-repellent property
3.3 “Liquid-like” fluorine-free surfaces with oil- repellent property
4 Application of fluorine-free surfaces with oil- repellent property
5 Conclusion and outlook
Qing Wang , Peng Li , Dawei Wu , Lu Jiang , Xinrui Fang , Haitao Niu , Hua Zhou . Research Progress for Design and Fabrication of Fluorine-Free Surfaces with Oil-Repellent Property[J]. Progress in Chemistry, 2025 , 37(2) : 255 -280 . DOI: 10.7536/PC240415
图4 3种微结构的制备工艺流程、SEM图片及液体悬浮在3种表面的状态:(1)热氧化、光刻和反应离子蚀刻(RIE)进行图像化、深度反应离子蚀刻(DRIE)、缓冲氧化物蚀刻去除顶部SiO2及涂覆C4F8等步骤制备传统的微米垂直结构,在 θY>90°时才能悬浮水;(2)热氧化、RIE、DRIE、各向同性Si蚀刻及涂覆C4F8等步骤制备重入结构,在θY<90°时可使油或有机溶剂悬浮;(3)热氧化、RIE、两次硅各向异性蚀刻、热氧化、RIE去除凹槽底部SiO2、DRIE及各向同性Si蚀刻等步骤制备双重入结构,在θY≈0°时可使任何液体悬浮[14]Fig. 4 Preparation processes, SEM images and liquid suspension states of three microstructures: (1) Vertical posts: thermally oxidized, patterned by photolithography and reactive ion etching (RIE), deep reactive ion etching (DRIE), buffered oxide etch bath to remove the top SiO2, C4F8 coated. θY > 90°, suspend water; (2) Re-entrant posts: thermally oxidized, RIE, DRIE, isotropic Si etching, C4F8 coated. θY < 90°, suspend oil or solvents; (3) Doubly re-entrant SiO2 posts: thermally oxidized, RIE, twice silicon isotropic etching, oxidized, RIE, DRIE, silicon isotropic etching. θY≈0°, suspend any liquid[14]. Copyright © 2014, The American Association for the Advancement of Science |
图5 无氟疏油织物:(1)蓖麻油、大豆油、矿物油、十六烷、葵花籽油、柴油、十四烷、十二烷、癸烷及辛烷在线性PDMS刷涂层织物表面的照片[17],(2)蓖麻油、橄榄油和菜籽油在改性尼龙夹克织物表面的接触角图片以及织物的SEM图像[19]Fig. 5 Fluorine-free oleophobic fabrics: (1) Images of castor oil, soybean oil, mineral oil, hexadecane, sunflower seed oil, diesel, tetradecane, dodecane, decane and octane on the surface of linear polysiloxane brush coated fabric[17]. Copyright © 2023 Published by Elsevier B.V. (2) Contact angle images of castor, olive and canola oils on modified nylon jacket fabric surfaces, with SEM images of the coated fabric structure[19]. Copyright © 2020, The Author(s), under exclusive licence to Springer Nature Limited |
表1 基于低表面能化合物和微纳米多尺度结构协同作用的无氟抗油污表面Table 1 Fluorine-free surfaces with oil-repellent based on the synergistic effect of low surface energy compounds and micro-nano multi-scale structures |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Glass | Vapor functionalization of as-deposited silica surfaces using hydrocarbon (octyltrichlorosilane), monomethyl silicone (trichloromethylsilane) or dimethyl silicone (dichlorodimethylsilane) functionalizing agent | Oily-water (hexadecane-water), ethanol-water mixture, cationic (cetyltrimethylammonium bromide, CTAB) and anionic surfactants (sodium dodecyl sulfate, SDS) | θ>150° | 15 |
| Aluminum alloy plate | Spray coating with organosilanes (TEOS and HDTMS) functionalized Al2O3 nanoparticles suspension | Ethylene glycol | θ>158°,α<5° | 16 |
| Fabric and glass | TEOS sol-gel (provide reactive silanol groups) and PDMS coating was prepared by hydrolysis and polycondensation of difunctional chlorosilane monomer via CVD | Castor oil, soybean oil, mineral oil, hexadecane, sunflower seed oil, diesel, tetradecane, dodecane, decane and octane | Fabric: θcastor oil =133.6°, θsoybean oil =92.5° | 17 |
| Fabric and glass | Dip-coating with TEOS sol-gel to prepare a silanol-coated fabric, followed by CVD with DCTMDSO | Castor oil, soybean oil and engine oil | Fabric: θcastor oil =119.0°, θsoybean oil =115.0°, θengine oil= 97.4° | 18 |
| Nylon fabric and metal mesh | Structural design (fabric), reactive groups were added (fabric: silanol group by using tetraethoxysilane, mesh: hydroxyl groups by oxygen plasma) and siloxane chemical modification (1,3-dichlorotetramethyldisiloxane vapor) | Canola oil, olive oil, hexadecane and castor oil | Fabric: θcanola oil /θolive oil /θcastor oil >90°, mesh: θhexadecane=98° | 19 |
| Metal and nylon fabrics | Oxygen plasma treatment to increase hydroxyl groups, PDMS brushes were then grown | Ethanol | Could reach around 120° | 20 |
| Quartz fiber | Silanized by APTES to impart positive charges, negatively charged SiNPs were then grafted, followed by PDMS grafting | Water-ethanol solutions (28~ 49 mN/m) | Could reach around 120° (28 mN/m water-ethanol) | 21 |
| Paper | Three-step coating: SNFC/CTS/modified polyvinyl alcohol | Castor oil | θ=103.9° (5 s), θ=87.9° (3 min) | 22 |
| Paper | CNF/PDMS+CNFmp | Castor oil | θ=91.8° | 23 |
| Paper | TOCNF was crosslinked with CA, CA-CNF was then modified with ODA | Castor oil | Paper: θcastor oil =118.4° | 24 |
图8 (1)液滴运动的温度响应过程[33],(2)液滴与纤维平行时液滴的电控液滴运动:分别施加3.5、0.5及2.5 V电压在倾斜角度为60°的表面控制水、氯化钾溶液及离子液体液滴的运动[36],(3)UV照射降解润滑油中的尼罗红染料[37]Fig. 8 (1) Temperature driven droplet motion process[33], Copyright © 2018 American Chemical Society. (2) Electrically controlled droplet motion in the direction parallel to the fiber: The motion of water, KCl solution and ionic liquid droplets was controlled by applying 3.5, 0.5 and 2.5 V voltages respectively on a surface with a tilt angle of 60°[36]. Copyright © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim. (3) Degradation of Nile Red on the surface by using UV illumination[37]. Copyright © 2017 Wiley- VCH Verlag GmbH & Co. KGaA, Weinheim |
表2 无氟抗油污SLIPSTable 2 Fluorine-free SLIPS with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Nanostructured silicon | Infused with the polar ionic liquid 1-ethyl-3-methylimidazolium methylsulfate | Toluene and cyclohexane | Δθtoluene=10.6°, Δθcyclohexane=4.7°. αtoluene=2.5°, αcyclohexane=1° | 29 |
| Ultrahigh-molecular-weight polyethylene and SiO2 nanoparticle mixtures (or other functional metal oxides, e.g. Fe3O4 and TiO2) | Functional molecules/inks were printed, then infused with lubricant (mineral oil/silicone oil) | Ethylene glycol, DMSO and N,N-dimethylformamide (DMF) | Slide readily | 30 |
| Zinc plate | Etching by formamide solutions to form nanostructures, PDMS grafted onto ZnO nanorods under UV (20 W, 354 nm), with residual non-bound silicone oil acting as a lubricant | Oleic acid | α≤4° | 31 |
| Glass | Casted by solutions of Jeffamine, DGEBA and PGMA-g-PDMS, then infused with or without silicone oil | Hexadecane, dodecane, decane and octane | αhexadecane=1°,αdodecane=1°,αdecane<1°,αoctane=2°;Δθ<1° | 32 |
| Glass | Spin-coating PS solution and paraffin lubricant onto the glass one after another | DMSO and ionic liquid | Droplet parallel to fiber (heating):θDMSO=37.3°, θionic liquid=58.1°, αDMSO=35.8°, αionic liquid=38.9°. Perpendicular direction:θDMSO=41.2°, θionic liquid= 63.4°, αDMSO=55°, αionic liquid=62.2° | 33 |
| Glass | Spin-coating PS solution and infused with paraffin wax/liquid paraffin wax/silicone oil | Formamide, ethylene, DMSO and ionic liquid | θformamide =92.1°, θethylene =81.8°, θDMSO=68.0°, θionic liquid=60.7°. αformamide=18.9°, αethylene=21.4°, αDMSO=24.7°, αionic liquid=27.2° | 34 |
| ITO substrate | Graphene oxide (GO) spin coated on ITO and reduced at 200 °C under vacuum to obtain rGO, infused with silicone oil/ionic liquid | Ethylene glycol | rGO and silicone oil surface:droplet parallel to fiber:θ=47.1°, α=52.3°, perpendicular:θ=53.2°, α=76.8°. rGO and ionic liquid surface:parallel:θ=22.8°, α=33.6°, perpendicular:θ=28.3°, α=42.2° | 35 |
| ITO substrate | Poly(3-hexylthiophene) spin coated on ITO, infused with silicone oil | Ionic liquid and 1,2-propylene glycol | Droplet (2 μL) parallel to fiber:αionic liquid=45.1°, αpropylene glycol=27.6°, perpendicular:αionic liquid= 72.7°, αpropylene glycol=50.9°. Droplet (10 μL) parallel to fiber:αionic liquid=13.6°, αpropylene glycol=5.8°, perpendicular:αionic liquid= 28.1°, αpropylene glycol= 9.4° | 36 |
| Glass | TiO2 nanoparticle pastes (or SiO2/mesoporous metal-oxide photocatalysts) coated and followed by sintering at 500 °C, PDMS chains were then grafted on the TiO2 surface under UV, residual PDMS layer acted as a lubricant | Ethylene glycol, methanol, methyl cyanide and fluorocarbon liquids (FC70, FC40 and FC72) | αethylene glycol=0.6°,αmethanol=2.3°,αmethyl cyanide= 1.1°,αFC70/αFC40/αFC72<0.5° | 37 |
表3 无氟抗油污凝胶Table 3 Fluorine-free gel with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Organogel | Mixing PDMS, organic liquid phase1 and organic liquid phase2 (e.g. isocetane and n-octadecyltrichlorosilane), then heated in a Teflon container/tray (perfluoroalkoxy copolymer resin)/glass vial/petridish to promote gelation | viscous emulsions: mayonnaise (vegetable oil as raw material), honey, ketchup, liquid glue, and worcester sauce | Slide readily on the organogel surface inclined at 30° | 38 |
| Glass, pipeline and copper mesh | Sodiumalginate homogeneous solution spin coated onto substrates, gelled in saturated CaCl2 aqueous solution to obtain the Ca2+/alginate hydrogel coating, then immersed into saturated LiCl aqueous solution, washed with ethanol and heated | Crude oil, engine oil and rapeseed oil | Slide readily on slightly inclined surface | 39 |
表4 支链烷基硅烷单分子膜的润湿性[41]Table 4 Wettability of monolayers comprising silanes with alkyl groups exhibiting branching structures[41]. Copyright © 1999, American Chemical Society |
| Functional groups | θadv/θrec of water/(°) | θadv/θrec of methylene iodide/(°) | θadv/θrec of hexadecane/ (°) |
|---|---|---|---|
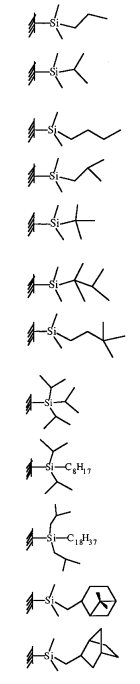 | 104/93 | 66/55 | 22/15 |
| 108/96 | 67/54 | 26/15 | |
| 105/94 | 64/60 | 26/20 | |
| 109/95 | 57/54 | 17/7 | |
| 104/98 | 60/57 | 26/21 | |
| 83/72 | 61/59 | 24/21 | |
| 118/98 | 67/53 | 22/10 | |
| 80/63 | 55/40 | 12/5 | |
| 73/55 | 52/40 | 14/5 | |
| 77/59 | 51/44 | 5/0 | |
| 97/91 | 55/47 | 5/0 | |
| 100/79 | 51/40 | 7/0 |
表5 无氟抗油污单层膜“类液体”表面Table 5 Fluorine-free “liquid-like” monolayers with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Oxidized aluminum- and titanium-covered Si substrates | Oxygen plasma treatment, vapor phase reaction with 1,3,5,7-tetramethylcyclotetrasiloxane (nitrogen atmosphere and heated at 80~180 ℃) | Hexadecane | Oxidized aluminum-covered Si substrate: Δθ=2°, Oxidized titanium-covered Si substrate: Δθ=3° | 40 |
| Silicon wafer | put in sodium dichromate and hydrogen peroxide solution to form silanol, and then silanized (reaction between surface silanols and monofunctional dimethylsilanes) | Hexadecane, methylene iodide | Δθhexadecane≥5°, Δθmethylene iodide≥2° | 41 |
图11 不同分子构型共聚物涂覆表面的油滑动行为(上方为4种共聚物涂层的分子构型示意图,下方为甲苯液滴滑动的图片):(1)IPDI/H2N-PDMS-NH2涂层(双循环PDMS共聚物刷),(2)IPDI/PDMS-(NH2)x交联涂层(双循环交联PDMS共聚物涂层),(3)IPDI/H2N-R-NH2涂层(双循环烷基共聚物刷),(4)IPDI/H2N-PDMS-NH2/H2N-R-NH2涂层(双循环烷基共聚物刷加上双循环PDMS共聚物刷)[47]Fig. 11 Sliding behavior of oil droplets on copolymer-coated surfaces with different molecular configurations (schematic diagrams of the molecular configurations of the four copolymer coatings at the top and the pictures of sliding toluene droplets at the bottom) : (1) IPDI/H2N-PDMS-NH2 coating (Two-cycle PDMS copolymer brushes), (2) IPDI/PDMS-(NH2)x cross-linked coating (Two-cycle cross-linked PDMS copolymer coating), (3) IPDI/H2N-R-NH2 coating (Two-cycle alkyl copolymer brushes), (4) IPDI/H2N-PDMS-NH2/H2N-R-NH2 coating (Two-cycle alkyl copolymer brushes plus two additional cycles of PDMS copolymer brushes)[47]. Copyright © 2017, American Chemical Society |
图12 (1)基材表面的硅烷醇和PDMS硅氧烷键的水解缩合[42],(2)乙烯基PDMS共价接枝到巯基改性玻璃制备过程[55],(3)在基材表面快速酸催化二甲氧基硅烷缩聚过程[56]Fig. 12 (1) Hydrolytic condensation of silanol and siloxane bonds in PDMS[42]. Copyright © 2011, American Chemical Society (2) Covalent grafting of vinyl-terminated PDMS to mercapto-modified glass[55]. Copyright © 2020, Springer Science Business Media, LLC, part of Springer Nature (3) Rapid acid-catalyzed polycondensation of dimethyldimethoxysilane on the substrate surface[56]. Copyright © 2016 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim |
表6 无氟抗油污聚合物刷“类液体”表面Table 6 Fluorine-free polymer brush type “liquid-like” surfaces with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Fabric, glass | TEOS sol-gel (provide reactive silanol groups) and PDMS coating was prepared by the hydrolysis and polycondensation of difunctional chlorosilane monomer via CVD | Castor oil, soybean oil, mineral oil, hexadecane, sunflower seed oil, diesel, tetradecane, dodecane, decane and octane | Glass: Δθcastor oil=7.3°, Δθsoybean oil=2.7°,Δθmineral oil=4.2°,Δθhexadecane=3.9°,Δθsunflower seed oil=5.1°,Δθdodecane=1.3°,Δθtetradecane=4.9° | 17 |
| Silicon wafer or metal-coated (Ti, Ni, Al) silicon wafer | Oxygen plasma treatment, drop silicone oil onto surface | Hexadecane, methylene iodide | Silicon wafer: Δθmethylene iodide≥2°, Δθhexadecane≥1° | 42 |
| Silicon wafer and glass | APTES treatment to form amino-functionalized surfaces (-NH2), IPDI and H2N-PDMS-NH2 coated alternately | Hexane, ethanol, acetone, dodecane, hexadecane, toluene, THF, DMF, DMSO, ethyl ether, octane, methanol, isopropanol, ethyl acetate, chloroform, dichloromethane, 1,4-dioxane | Δθ<4° | 47 |
| Ceramic, vitreous china and carbon steel | Rinsed with isopropanol and deionized water, sprayed by dimethyldimethoxysilane and wetted by silicone oil | Synthetic human faeces (composed of yeast, psyllium, peanut oil, miso, polyethylene glycol, calcium phosphate, cellulose and water) | Repel liquids, bacteria and viscoelastic solids with dynamic viscosities spanning over nine orders of magnitude. Slip without a trace. | 48 |
| Glass, metal, plastic, silicone rubber, silicon wafer, stainless-steel and acrylic sheet | Hydrosilylation reaction between MV-PDMS and SiH-PDMS | Cooking oil and hexadecane | Cooking oil: slip without a trace Δθhexadecane=5°~10°, αhexadecane=4°~9° | 49 |
| Oxidized Si substrate | Exposed to 1,3,5,7-Tetramethylcyclotetrasiloxane vapor, immersed in vinyl-terminated PDMS and Karstedt’s catalyst hexane solution (Pt-catalyzed hydrosilylation) | Hexadecane, dodecane and decane | PDMS chains with molecular weight (MW) 6000: Δθ<5°. PDMS chains with MW 117000: Δθ<20° | 50 |
| Glass | POII-g-PDMS Synthesis (PDMS-OH +oxalyl chloride→PDMS-COCl, PDMS-COCl+PO→ PO-g-PDMS, PO+2-isocyanatoethyl methacrylate→POII-g-PDMS). TM Synthesis (dibutyltin dilaurate catalyst+ HEMA+ HDIT). Synthesis of Mixtures of TM and DM-PDMS (PDMS-OH+HDIT+ hydroxyl-to-isocyanate+ HEMA+ dibutyltin dilaurate catalyst). F1: DM-PDMS and TM mixture, F2: POII and POII-g-PDMS, F3: TM and POII-g-PDMS. | Hexadecane | F2: α=2.4°, F3: α=2.8° | 51 |
| Glass | PEMA+PDMS-NH2→PEMA-g-PDMS, PEMA-g-PDMS was dissolved in acetone, CEOS was dissolved in N-methyl-2-pyrrolidone, mix two reagent and cast onto the glass pretreated with air plasma, solvent evaporated and heat cure coating | Hexadecane, dodecane, decane, diiodomethane and methanol | αhexadecane/αdodecane /αdecane<5°, αdiiodomethane/αmethanol<13° | 52 |
| Glass and PAES-g-PDMS film | The uniform grafting via click chemistry of liquid PDMS onto PAES | Hexadecane, dodecane, decane, octane, methanol and ethanol | αhexadecane/αdodecane /αdecane/αoctane<4°, αmethanol/αethanol<13° | 53 |
| Silicon wafer | 1,3,5,7-tetramethylcyclotetrasiloxane+ vinyl-terminated PDMS+ Karstedt’s catalyst (Ptcatalyzed hydrosilylation) | Diiodomethane, hexadecane and dodecane | Δθ<5°, α<10° | 54 |
| Silicon wafer | APTES treatment to form amino-functionalized surfaces (-NH2), IPDI and H2N-PDMS-NH2 coated alternatively, print FITC inks | 1,4-dioxane, toluene, THF, acetone, ethanol, ethyl acetate, chloroform, hexane, ethyl ether and dichloromethane | Surface with low or medium FITC patterning density: Δθ<5°. Surface with high FITC patterning density: Δθ<10° | 44 |
| Glass | Activated in sodium hydroxide solution to introduce —OH on glass surface, occur dehydration condensation reaction between —OH and MPTS, then vinyl-terminated PDMS grafted onto the mercapto-modified glass via thiol-ene click reaction | Formamide, DMSO and ethanol | α<5° | 55 |
| Silicon wafer | Oxygen plasma treatment, immersed in dimethyldimethoxysilane and sulfuric acid isopropanol solution (acid-catalyzed graft polycondensation of dimethyldimethoxysilane) | Diiodomethane, toluene, hexadecane, cyclohexane, decane and hexane | Δθ≤1° | 56 |
| Paper | Oxygen plasma treatment, vapor-deposited using 1,3-dichlorotetramethyldisiloxane | Ethanol, hexadecane, olive oil, castor oil and ethylene glycol | Δθ<6° | 57 |
表7 无氟抗油污PDMS复合涂层表面Table 7 Fluorine-free PDMS composite coating surfaces with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Glass and PET film | GPOSS+PDMS-NH2→GPOSS+GPOSS-g-PDMS mixture, mixing GPOSS+GPOSS-g-PDMS mixture, pristine GPOSS and photoinitiator in acetonitrile and propylene carbonate, cast onto asubstrate, solvent evaporated and precursor films were photolyzed | Diiodomethane, methanol, hexadecane and decane | αdiiodomethane=9°, αmethanol=8°, αhexadecane=5°, αdecane=3°. ΔθDiiodomethane=5°, Δθmethanol=5°, Δθhexadecane=4°, Δθdecane=10° | 58 |
| Glass, Teflon, PET, aluminum and steel substrates | Thiol-ene click reaction between POSS and SBS under UV irradiation, dispersing OH-PDMS-OH within the crosslinked network between POSS and SBS | Hexadecane, dodecane, dichloromethane, ethanol, pump oil and cooking oil | Slide readily, αhexadecane=5.5° | 45 |
| Glass | PU coating was partially crosslinked on the substrate, then PDMS-NH2 solution was applied as a top-layer, nanofillers (e.g., nanoclay, cellulose nanocrystals and GO) could be incorporated | Hexadecane | Without nanofillers: αhexadecane=11°. With nanofillers: αhexadecane=10° | 60 |
| Paper | Double coating: PU and PDMS | Oil | Slip without leaving a trace | 61 |
| Various substrates | PDMS-COCl reacted with polyol component to yield a graft copolymer, added by HDIT, cast onto glass plates, solvent evaporated and thermally cured | Fingerprint liquid (consisting of lactic acid, acetic acid, sodium chloride, sodium hydrogen phosphate,1-methoxy-2propanol, hydroxy-terminated PDMS and deionized water), hexadecane, dodecane, decane, methanol, ethanol and diiodomethane | Slide readily | 62 |
| Glass | Casting solution comprising HDIT, polyol, polyol grafted PDMS and free silicone oil/SO mixture onto the substrate | Diiodomethane, hexadecane, THF, dodecane, decane, octane, methanol, ethanol and perfluorooctane | α<10° | 63 |
| Glass | Blocked polyisocyanate and graft copolymer polyol-g-PDMS were codispersed in dipropylene glycol monomethyl ether and water solution, cast onto the substrate, solvent evaporated and thermally cured | Diiodomethane, hexadecane, dodecane and decane | Surfaces contained 9.7% of PDMS: αdiiodomethane=5°, αhexadecane=2°, αdodecane=2°, αdecane=1° | 64 |
| Glass and wood | PEI graft (g) PDMS, mixing PEI-g-PDMS and DGEBA in butanone (with or without hardener mixture), cast on the substrate and thermally cured | Cooking oil, pump oil, oil-based paints and ink | Repellent to oils, oil-based paints and ink readily shrank | 65 |
| Glass | HDIT+PDMS-NH2→PDMS-NCO solution,Mixing BADGE and Jeffamine D230 to prepare EP coating, cast onto glass (partial cross-linking), applying PDMS-NCO onto EP coating and thermally cured, nanofillers (e.g., nanoclay, cellulose nanocrystal and GO) could be incorporated | Hexadecane | EP-PDMS: α=9°, nanoclay-EP-PDMS: α=7°, cellulose nanocrystal-EP-PDMS: α=6°, GO-EP-PDMS: α=8° | 66 |
| Glass | Mixing MMA, BA and AA to prepare the preemulsion, HPMA participated in the particle growth stage of the polymerization, yielding PPH, HEMF offered strong connectivity among PPH and SSH via crosslinking, cast onto the substrate and thermally cured | Diiodomethane, hexadecane, ethanol, cooking oil, pump oil and crude oil | αdiiodomethane=2°, αhexadecane=3°, αethanol=4°,αcooking oil=9°, αpump oil=7°,αcrude oil=7° | 67 |
表8 无氟抗油污的溶胶-凝胶杂化涂层Table 8 Fluorine-free sol-gel hybrid coatings with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Glass and silicon wafer | ZTP and BSA/LSA react at 150 ℃ to form a precursor, added by glacial acetic acid and isopropanol, then spin-cast onto UV/ozone-cleaned substrates | Hexadecane | Zr:BSA Hybrid Film: Δθ=4.9°, α=10.4°. Zr:LSA Hybrid Film: Δθ=8.9°, α=11.4° | 68 |
| Glass, polyimide film and stainless-steel | MTES was hydrolysed and condensed in HCl-ethanol solution, spin-coated onto substrates, dried at 100 ℃ to promote condensation of siloxane networks (acid catalysed sol-gel reaction) | Hexadecane, dodecane and decane | θhexadecane=35°, θdodecane=24°, θdecane=17°. α<5° | 69 |
| Glass, Si, polycarbonate and poly(methyl methacrylate) substrates | Mixing DTES and TMOS in an ethanol/ hydrochloric acid solution, spin-coated onto UV/ozone-cleaned substrates | Diiodomethane, hexadecane, oleic acid, soybean oil, toluene, p-xylene, turpentine oil, n-dodecane, n-decane, and ethanol | Δθ=0~5°, α=2.4~8.8° | 70 |
| Glass and silicon wafer | Sol-gel method: HCl+ ethanol+ tetramethoxysilane +8 different alkyltriethoxysilanes with a range of alkyl chain lengths (CnTES, n = 3, 6, 8, 10, 12, 14, 16 and 18), spin-cast on UV/ozone cleaned substrates | Hexadecane, dodecane and decane | n=3~10, α<5°. n=12, α<10°. n=12~14, α>10° or pinning | 71 |
| Glass and water-free synthetic fused silica plates | Sol-gel reaction: mixing DTES and TMOS in an ethanol/hydrochloric acid solution, condensation of silanol groups to form polymer, spin-coated onto UV-ozone-cleaned substrates | DMF, toluene and isopropanol | The dielectric constant of the probe liquids is less than 30: Δθ<3° | 72 |
图18 (1)液滴在常规超疏液和双移动超驱避表面上的迁移示意图,(2)双移动超驱避表面的扫描电镜图,(3) PFPE链接枝到表面微柱结构的示意图,(4)Wenzel态液滴的侧向附着力示意图,(5)黏附力原理图[73]Fig. 18 (1) Schematic of droplet migration on a conventional super-repellent surface and a dual-mobility super-repellent surface, (2) SEM image of the dual-mobility super-repellent surface, (3) Diagram showing PFPE chains grafted onto the surface micro-pillar structure, (4) Schematic of lateral adhesion force for a Wenzel-state droplet, (5) Diagram of adhesion force principles[73]. Copyright © 2024 Wiley‐VCH GmbH |
表9 无氟抗油污的纳米级粗糙“类液体”涂层表面Table 9 Fluorine-free nanohierarchical rough "liquid-like" surfaces with oil-repellent |
| Substrates | Preparation strategy | Testing oils | Oleophobicity | Ref |
|---|---|---|---|---|
| Silicon wafer | SiO2 particle-based surfaces were prepared using liquid flame spray, spray coating, and soot deposition from a paraffin candle, followed by functionalization with dichlorotetramethyldisiloxane via CVD to form surface-tethered linear PDMS chains | Ethylene glycol, diiodomethane, water-ethanol solutions | θ>150°, α<10° | 74 |
| Glass | APTES-functionalized glass, layer-by-layer growth of MOFs, infused with alkyl silane | Vegetable oil, butanone, ethanol, methanol, acetone, 1-butanol, 1-decanol, glycol, cyclohexanol, and 1,2-butanediol | Δθ=4°~15° | 75 |
图20 黄色荧光指纹粉在无氟抗油污玻片表面:(1)水洗前和(2)水洗后;未涂布玻片上的指纹:(3)水洗前和(4)水洗后[68],(5)无氟抗油污纸基材料能够含有水(蓝色)、橄榄油(黄色)和乙醇(红色)48 h而不吸收液体[57],(6)表面图案化过程[44]Fig. 20 Pictures of fingerprints detected with yellow fluorescent fingerprint powder on fluorine-free glass slide surfaces with oil-repellent: (1) before and (2) after washing with water; Pictures of fingerprints on uncoated slide surfaces: (3) before and (4) after washing[68]. Copyright © 2013, American Chemical Society (5) Fluorine-free paper-based microtiter plate with oil-repellent effectively retained deionized water (blue), olive oil (yellow), and ethanol (red) for 48 h without any liquid adsorption[57]. Copyright © 2020, American Chemical Society (6) Schematic illustration of surface patterning process[44]. Copyright © The Royal Society of Chemistry 2017 |
图21 水(染蓝色)和合成粪便分别在最先进的商用疏水釉面厕所马桶(左)和涂覆LESS的厕所马桶表面(右)的驱避性对比[48]Fig. 21 Comparison of repellency of water (dyed blue) and synthetic feces between a state-of-the-art commercial hydrophobic glaze-coated toilet (left) and a LESS coated toilet bowl surface (right)[48]. Copyright © 2019, The Author(s), under exclusive licence to Springer Nature Limited |
图22 (1)水在涂覆抗油污涂料的木板上滑动,(2)十六烷在涂覆涂料后的不锈钢盘上滑动,(3)水在涂覆涂料后的棉织物上滚动,(4)盐酸液滴在涂覆和未涂覆涂料的铁板状态对比,(5)在半涂覆手机表面涂料涂覆和未涂覆的表观对比[62]Fig. 22 (1) Water droplet slid on a coated wooden plate with oil-repellent, (2) Hexadecane droplet slid on a coated stainless-steel disk, (3) Water slid on a coated cotton fabric, (4) A comparison of HCl droplets on coated and uncoated iron plate, (5) Apparent comparison of coated and uncoated coatings on a half-coated cell phone surface[62]. Copyright © 2015 WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim |
图23 加载热致变色油墨的拒液涂层:(1)60 ℃的水(绿色)在表面滑动(颜色变化);20 ℃的水(黄色)在表面滑动(没有引起颜色变化),(2)“COOL”红色图案在60 ℃水中消失,在20 ℃水中重现,(3)油墨在6 s内通过丙酮超声去除[30]Fig. 23 The liquid repellent coating loaded with thermochromic ink: (1) Water droplet (60 ℃,green) slid on the surface (color change); Water droplet (20 ℃,yellow) slid on the surface (no color change), (2) Red pattern “COOL” disappeared in water at 60 ℃ and reappeared in water at 20 ℃, (3) Inks were removed by acetone ultrasound within 6 s[30]. Copyright © The Royal Society of Chemistry 2018 |
图24 (1~3)不同基材上无氟抗油污涂层的抗原油黏附性:(1)金属、(2)木材和(3)陶瓷[67],(4~6)抗油污水凝胶:(4)黏有菜籽油的水凝胶表面只需用水浸泡即可完全去除,(5)水凝胶的油水分离实验,(6)水凝胶涂覆铜网可以将液体聚合物与水分离[39]Fig. 24 (1~3) Anti-crude oil adhesion of fluorine-free coating on different substrates: (1) metal, (2) wood and (3) ceramic[67]. Copyright © 2019 American Institute of Chemical Engineers. (4~6) Hydrogel with oil-repellent: (4) sticky rapeseed oil was detached by water immersion, (5) oil-water separation experiment, (6) separation liquid polymer from water by hydrogel coated copper mesh[39]. Copyright © 2022, Science China Press and Springer-Verlag GmbH Germany, part of Springer Nature |
表10 无氟抗油污表面的应用Table 10 Application of fluorine-free surfaces with oil-repellent Property |
| Substrates | Application | Ref |
|---|---|---|
| Various substrates | Oil resistant/self-cleaning/stain resistant | 19,30,39,45 |
| Various substrates | Moisture/dampness resistance | 55 |
| Various substrates | Anti-corrosion | 62 |
| Glass, silicon, ceramics, carbon steel | Anti-graffiti/anti-fingerprint/anti-smudge/anti-adhesion (new smooth materials, e.g. toilets) | 32,34,39,48,62,68,71 |
| Glass and Teflon | Anti-icing (smart Windows, handheld displays, goggles and windshields) | 30,38,39,75 |
| - | Sensor | 30 |
| Paper, glass and silicon wafers | Food industry (bags, beverage cans and glassware) | 76,77 |
| Glass | Biomedical industry (packaging bags, non-polluting medical equipment) | 30,44,45,78 |
| Glass | Outdoor devices and chips | 10 |
| Glass, silicon, polyimide film and stainless steel | Optical components, touch screen equipment, internal engine/pump, kitchen equipment | 68,69 |
| Glass | Biochips and microreactor systems | 33 |
| Glass | The extraction, transportation, storage and processing of petroleum | 67 |
| - | Oil funnel, microtitration plate | 57 |
| Glass, silicon, steel, flexible plastic, aluminum foil, PTFE pipe, stainless steel mesh and ITO substrate | The drag reduction, handling, non-destructive transport, directional transport, collection and detection of water fluid/microfluidic (pipeline) | 36,44 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
Wenzel, Robert N. Trans. Faraday Soc., 1936, 28 (8): 988.
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
/
| 〈 |
|
〉 |