

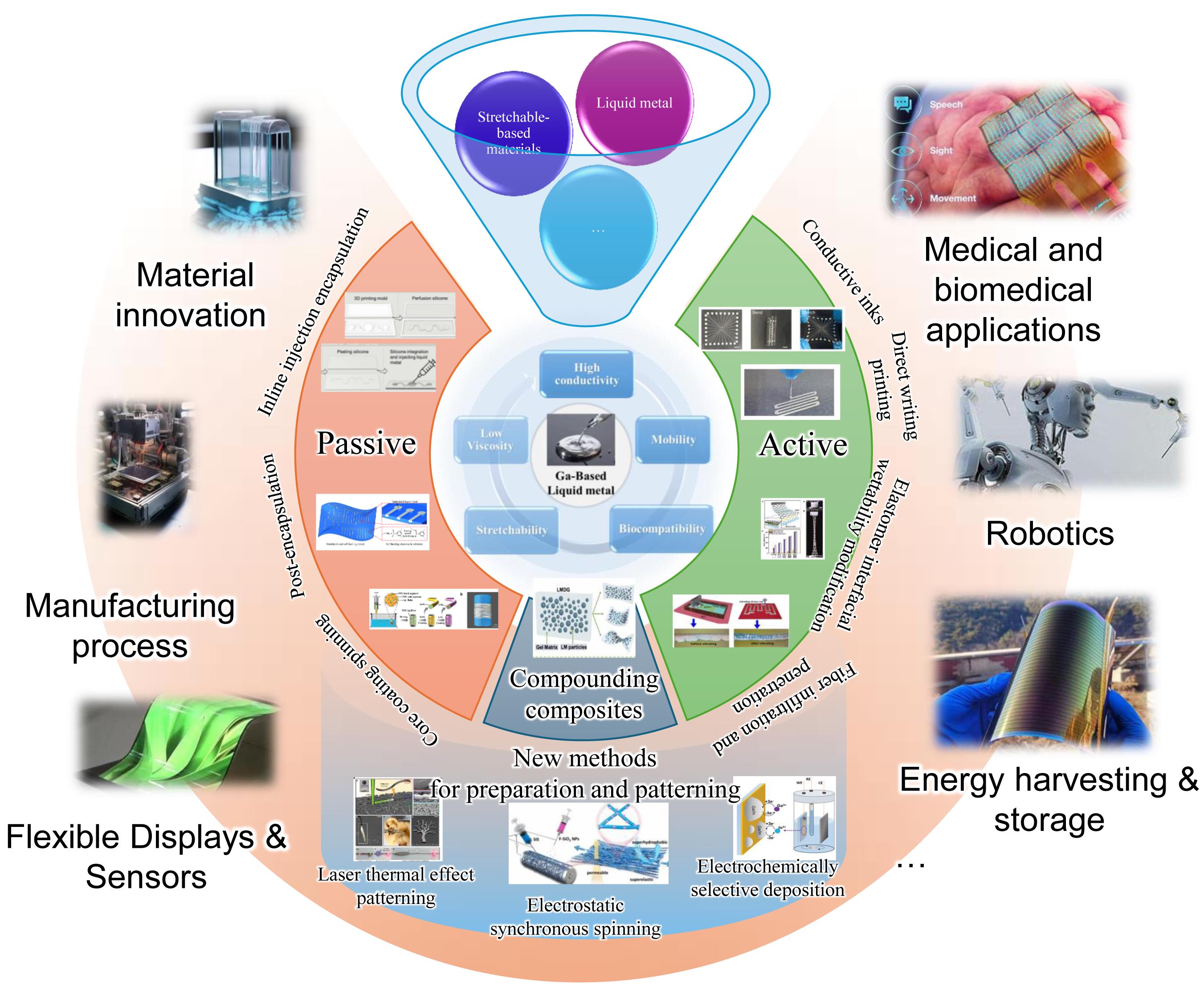
Liquid Metal-Based Stretchable Conductive Composites
Received date: 2024-05-13
Revised date: 2024-08-15
Online published: 2024-09-06
Nowadays stretchable electronic devices have become a hot research topic in the field of information electronics because of their excellent mechanical and electrical properties. As the high-speed electron transmission channel in stretching electronic devices, stretchable conductive materials play a crucial role in realizing the functions of stretching electronic devices. Liquid metal has become a hot research object in the field of stretchable conductive composites in recent years because of its intrinsic flexibility and excellent conductivity. Liquid metal is a room temperature liquid conductive material, which exhibits excellent stretchability and tunability due to its inherent high conductivity, fluidity, and ductility. Liquid metal-based stretchable conductive composites preparation and patterning techniques have been reported and many stretchable devices with excellent combination of mechanical and electrical properties have been prepared. In view of the general structural characteristics of liquid metal-based stretchable composites, the key to the preparation is how to solve the interfacial non-impregnation problem caused by the physical property differences between different materials. Therefore, starting from the common types of composites, this paper firstly briefly introduces the components and physical properties of liquid metals generally used, as well as the stretchable polymer matrix materials usually employed. Then, the composite methods of conductive materials and elastomer materials in liquid metal-based electrodes are reviewed from the two ways of "passive" and "active" to deal with the problem of non-wetting at the interface, as well as the blending and dispersion method and the new modification method. Finally, the latest research progress is introduced, and the current status of liquid metal research is summarized. Future development and potential problems to be faced are also discussed.
1 Introduction
2 Liquid metal-based flexible device material composition
2.1 Liquid metal and its composite materials
2.2 Flexible substrate material
3 Preparation method of liquid metal-based flexible conductive composites
3.1 Passive internal embedding method
3.2 Active surface structure modification method
3.3 Direct blending composite method
3.4 New methods for the preparation and patterning of liquid metal electrodes
4 Conclusion and outlook
Zaiyang Zheng , Huibin Sun , Wei Huang . Liquid Metal-Based Stretchable Conductive Composites[J]. Progress in Chemistry, 2025 , 37(3) : 295 -316 . DOI: 10.7536/PC240516
| Mp (℃) | Bp (℃) | Viscocity (10-7 m2/s) | Surface tension (N/m) | Conductivity (106 S/m) | Heat conductance coefficient (W·m/K) | |
|---|---|---|---|---|---|---|
| Hg | -38.8 | 357 | 13.5 | 0.5 | 1.0 | 8.34 |
| Ga | 29.8 | 2204 | 3.24 | 0.72 | 3.7 | 29.4 |
| EGaIn | 15.5 | 2000 | 2.7 | 0.624 | 3.4 | 42.2 |
| Galinstan | -19 | 1300 | 2.98 | 0.533 | 3.1 | 44.8 |
图1 (a) Schematic Illustration of the Fabrication Process of Flexible Microfluidic Sensors[110]; (b) Optical Images of Sensors with Straight and Sinusoidal Channels[110]; (c) Photographs of the Sensor under Different Stretching Strains and the Relative Change in Resistance of the Enhanced Sinusoidal Sensor when Stretched from ε = 0 to ε = 320%[110]; (d) Schematic Illustration of the Fabrication Process of Microchannels in Ecoflex[47]; (e) Fabrication Process of msw-TENG[111]; (f) Tensile Diagram of Ecoflex Film, Inset Shows the Conductive Wire Maintaining Conductivity under Stretching[47]; (g) Structure and Actual Image of Flexible Electrodes Prepared by Pan et al.[112]; (h) Actual Image of Flexible Breadboard Prepared by Kim et al.[113]Fig.1 (a)Schematic illustration of the fabrication process of the flexible microfluidic sensor[110];(b)Optical images of sensors with the straight channel and wave-shaped channel[110];(c)Photographs of the sensor which was stretched under different tensile strains and plot of the relative change in the resistance of the enhanced wave-shaped sensor when stretched from ε = 0 to ε = 320%[110];(d)Schematic diagram of the fabrication process of microchannels in an Ecoflex[47];(e)The fabrication process of the msw-TENG[111];(f)Ecoflex film was stretched. The inset diagrams illustrate that the liquid wires maintain conductivity in the stretched state[47];(g)Structure and physical diagram of flexible electrode prepared by Pan et al.[112];(h)Actual diagram of flexible breadboard prepared by Kim et al.[113] |
图2 (a) Steps to Create Liquid Metal Electrodes Embedded in Elastomers[51]; (b) Bendable and Stretchable Liquid Metal Interdigitated Electrodes Embedded in Elastomers and Capacitance at Different Curvature Diameters[51]; (c) Fabrication Steps of Stretchable Multilayer LMWPS; (d) Structure and Fabrication Process of Embedded Self - Healing Conductors[122]; (e) Encapsulation of Gallium Surface Oxidation 3D Micro - Nano Wrinkle Structures[123]; (f) LM - TENG Based on Liquid Metal Prepared by Munirathinam et al.[124]Fig.2 (a)The procedure used to create the elastomer-embedded liquid metal electrode[51];(b)The elastomer-embedded liquid metal interdigital electrodes can be curved and stretched, capacitance under different diameters of curvature[51];(c)Fabrication process of the stretchable multilayer LMWPS[121];(d)Structure and fabrication process of the embedded self-healing conductor[122];(e)Gallium surface oxidized 3D micro-nano wrinkle structure encapsulation[123];(f)A liquid metal-based triboelectric nanogenerator (LM-TENG) prepared by Munirathinam et al[124] |
图3 (a) Schematic Illustration of the Core-Sheath Fiber Composed of Polyurethane and Liquid Metal Fabricated by Coaxial Wet Spinning Strategy, with the Inset Showing Continuous Fiber Collection[33]; (b) Digital Image of the Integrated Glove with Stretch Sensing, Reversible Pressing Type Switching, and Low-Power Electrothermal Management Functions[33]; (c) Schematic Illustration of the Fabrication Procedure of Textile-Based TENG (t-TENG)[128]; (d) Lee et al.'s 2D Capacitive Sensor Made with Liquid Metal Core Ultra-Stretchable Elastic Fiber[130]; (e) Programmable LM Fiber Fabricated by Ma et al.[132]; (f) Stretchable Conductive Fiber with Binary Rigid-Soft Conductive Components and Dynamic Compensation Conductivity Capability Fabricated by Zhang et al.[131]Fig.3 (a)Schematic diagram of fabrication of core-sheath fibers composed of PU and liquid metal via a coaxial wet-spinning strategy. The inset image exhibits continuous fiber collection[33];(b)Digital images of the integrated glove with stretch sensing, reversible pressing-type switching and low-power electrothermal management functions[33];(c)Schematic illustration, fabrication procedure of the textile-based TENG (t-TENG)[128];(d)a two-dimensional capacitive sensor from liquid metal-core super-stretched elastane fibers fabricated by Lee et al.[130] ;(e)Programmable LM fibers manufactured by Ma et al[132];(f)Stretchable conductive fibers manufactured by Zhang et al. with binary rigid and soft conductive components and dynamic compensation conductivity[131] |
图4 (a) Schematic Illustration of SEA Fabrication. EGaIn Nanoparticles Prepared by Probe Sonication and Screen Printing on PET Substrate, After Transfer to PDMS Substrate, SEA Prepared by Pt Deposition and Si3N4 Passivation Layer Coating[83]; (b) Snapshots of SEA with High Flexibility (Middle) and Stretchability (Right)[83], Scale Bar: 5 mm; (c) Schematic Illustration of SCA and Stretchable Light-Emitting Diode (LED) Screen Fabrication[54]; (d) A Fully Self-Powered Mechano-Luminescent Triboelectric Sensor Based on Micro-Nanostructured Mechano-Luminescent Elastomer Constructed by Zhou et al.[148]; (e) A General Approach Proposed by Carneiro et al. for Fabricating Thin-Film Biostickers for High-Resolution Electrophysiological Monitoring[149]Fig.4 (a)Schematic illustration of fabrication of the SEA. EGaIn NPs were prepared by probe sonication and screen printed on PET substrate. After being transferred to PDMS substrate, the SEA was fabricated by Pt deposition and Si3N4 passivation layer coating[83];(b)Snapshots of the SEA with high flexibility (middle) and stretchability (right)[83]. Scale bar: 5 mm;(c)Schematics of the preparation of SCAs and stretchable light emitting diode (LED) screen[54];(d)a fully self-powered mechanical luminescent triboelectric sensor based on micro-nano structured mechanical luminescent elastomers constructed by Zhou et al.[148];(e)A general method for the preparation of thin-film biostickers for high-resolution electrophysiological monitoring proposed by Carneiro et al[149] |
图5 (a) Chip Integrated Circuit Manufacturing Process[157]; (b) Fabrication Steps of Printing Multilayer Circuits[58]; (c) Battery-Free Multilayer NFC Circuit[58]; (d) Soft Matter Circuit with Integrated Sensors, Microprocessors, and LED Display for Temperature Measurement, Skin, and Circuits with Multiple LEDs[157]; (e) Samples Cut and Healed under 900% Stretching Strain Test and Magnified Image of the Sample before Fracture[157]; (f) Full Process of PVA-LM Ink: Fabrication, Printing, Recycling, and Various Patterns Printed Using PVA-LM Ink[56]; (g) Process of Printing Liquid Metal (Top) and PVA-LM Ink (Bottom) on PET Film[56]; (h) High-Resolution Printing of Liquid Metal[57]; (i) Three-Dimensional Reconstruction of Liquid Metal[57]; (j) Zu et al. Formulated High-Performance Liquid Metal Conductive Ink and Achieved Direct Writing Printing[158]Fig. 5 (a)Process for chip-integrated circuit fabrication[157];(b)Fabrication steps for printing multi-layer circuits[58];(c)A battery-free multi-layer NFC circuit[58];(d)Soft matter circuits with integrated sensors, microprocessors and LED displays for temperature measurements, skin and circuits with multiple LED[157];(e)A dully cut and healed sample under tensile strain test of 900%, and the magnified image of the sample prior to breaking[157];(f)Whole process of the PVA-LM ink: fabrication, printing, and recycling,diverse patterns printed using the PVA-LM ink[56];(g)Printing process of the liquid metal (above)and PVA-LM ink (under)on a PET film[56];(h)High-resolution printing of liquid metals[57];(i)Reconfiguration of liquid metals into 3D structures[57];(j)a high-performance liquid metal conductive ink prepared by Zu et al. and direct print printing[158] |
图6 (a) Schematic Illustration of the Process for Creating a Smooth and Uniform Liquid Metal Film as a Reflective Electrode[167]; (b) Schematic Diagram of Manufacturing Steps[168]; (c) Dong et al.'s Printing of Stretchable Liquid Metal (EGaIn) Circuits on ZnO NPs - Anchored Ultrafine Fibers[169]; (d) Elastic Origami Electrode Based on Liquid Metal (LM - eKE) Fabricated by Choi et al.[170]; (e) Liquid Metal Electrode with Negative Piezo - Resistivity Prepared by Han et al.[171]Fig. 6 (a)Schematic illustration of the process flow to create a smooth and uniform liquid metal film as a reflective electrode[167];(b)Schematic illustration of the fabrication steps[168];(c)stretchable liquid metal (EGaIn) circuits printed on ZnO NPs-anchored microfibers by Dong et al.[169];(d)Liquid metal-based elastic origami electrode (LM-eKE) prepared by Choi et al.[170];(e)A liquid metal electrode with negative piezoresistivity prepared by Han et al[171] |
图7 (a) Schematic Diagram of Printing POLMs on NLF via Screen Printing, Optical Micrographs of POLMs/NLF Before and After Air Compressor Driven Rod Vibration[63]; (b) Pattern Drawing Using POLM[63]; (c) Schematic Diagram of PDMS-LM/Textile Fabrication[62]; (d) Photos of PDMS-LM/Fabric Before and After Mechanical Compaction[62]; (e) SEBS Ultrafine Fiber Penetrated with Liquid Metal Electrode Prepared by Dong et al.[175]; (f) Stretchable Soft Capacitive Sensor Capable of Distinguishing Various Hand Motions, Prepared by Bhuyan et al. Through Graphite Nanofiber (GNF) Filler Dispersed in Polydimethylsiloxane (PDMS) Substrate[176]Fig. 7 (a)Schematic illustration processes of printing POLMs on the NLF by screen printing and optical micrographs of POLMs/NLF before and after vibrating with an air-compressor-driven rod[63];(b)Patterning using the POLMs[63];(c)Schematic diagram for the fabrication of the PDMS-LM/Textile[62];(d)Digital photographs of the LM/Textile before and after mechanical compaction[62];(e)SEBS ultrafiber permeable liquid metal electrode prepared by Dong et al[175]; (f)a stretchable soft capacitive sensor capable of distinguishing various hand movements from graphite nanofiber (GNF) fillers dispersed in a polydimethylsiloxane (PDMS) substrate fabricated by Bhuyan et al.[176] |
图8 (a) Dielectrophoresis (DEP) Assembly of Liquid Metal (EGaIn) Microfibers[186]; (b) Schematic Illustration of the Preparation Method for SBS&LM@MUA[187]; (c) Kirigami Electrodes of Blended Liquid Metal Fabricated by Li et al.[188]; (d) Self-Healable Flexible Liquid Metal Electrodes Prepared by Pei et al.[189]; (e) Liquid Metal Droplet-Dispersed Hydrogels Prepared by Zhang et al.[190]; (f) Liquid Metal Droplet-Dispersed Gels Prepared by Bhuyan et al.[191]Fig.8 (a)Dielectric electrophoresis (DEP) assembly of liquid metal (EGaIn) microfilaments[186];(b)Schematic diagram of the preparation method of SBS&LM@MUA[187];(c)A blended liquid metal decoupage electrode manufactured by Li et al[188];(d)Self-healing flexible liquid metal electrode prepared by Pei et al.[189];(e)Liquid metal droplet dispersed hydrogel prepared by Zhang et al.[190];(f)Liquid metal droplet dispersion gel prepared by Bhuyan et al.[191] |
图9 (a) Kim et al. Fabricated Freestanding Patterned Liquid Metal Thin Film Conductors (FS-GaIn) by Laser Processing and Direct Patterning Trace Etching [195]; (b) Cho et al. Developed Laser-Induced Photothermal Reaction BMC Patterning [196]; (c) Luo et al. Developed Laser-Assisted Fabrication Designed Fully Soft Self-Powered Vibration Sensor (SSVS) [197]Fig.9 (a)Free-Stand-Alone Patterned Liquid Metal Film Conductors (FS-GaIn) by Laser Processing and Direct Patterned Traces through etching by Kim[195];(b)Laser-induced photothermal reaction BMC patterning developed by Cho et al[196];(c)A fully soft self-powered vibration sensor (SSVS) designed for laser-assisted manufacturing developed by Luo et al.[197] |
图10 (a) A Highly Robust Stretchable Electrode (NHSE) Based on Nanoliquid Metal Fabricated by Cao et al. Using Electrospinning [25]; (b) A Lightweight and Highly Conductive Composite Embedded with Liquid Metal Fiber Networks Synthesized by Ma et al. [198]; (c) An Omnidirectionally Superelastic, Permeable, and Superhydrophobic Microfiber Membrane (SPSM) Prepared by Li et al. Through Simultaneous Electrospinning of Styrene-Isoprene (SIS) Block Copolymer and Electrospraying of Fluorinated SiO2 Nanoparticles [199]Fig.10 (a)a nano-liquid metal-based highly robust stretchable electrode (NHSE) using electrospinningfabricated by Cao et al.[25];(b)A lightweight, highly conductive composite material embedded in a network of liquid metal fibers synthesized by Ma et al.[198];(c)Omnidirectional hyperelastic permeable and superhydrophobic microfiber membranes (SPSMs) prepared by Li et al. by simultaneous electrospinning of styrene-isoprene (SIS) block copolymers and electrospraying of fluorinated SiO2 nanoparticles[199] |
图11 (a) Monnens et al. Achieved High-Density Circuit Integration by Electrodepositing EGaIn[200];(b) Santi et al. Modified GO@EGaIn via an Electrochemical Cell for Supercapacitor Fabrication[201]Fig.11 (a)Monnens et al. performed high-density integration of circuits by electrodepositing EGaIn[200];(b)Santi et al. modified GO@EGaIn by electrochemical cells to fabricate supercapacitors[201] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
|
| [196] |
|
| [197] |
|
| [198] |
|
| [199] |
|
| [200] |
|
| [201] |
|
/
| 〈 |
|
〉 |