

Degradation of Antibiotics Using ZVI/H2O2 Fenton-Like Technology
Received date: 2024-05-11
Revised date: 2024-06-19
Online published: 2024-07-01
Supported by
National Natural Science Foundation of China(42277003)
ZVI/H2O2 Fenton-like technology overcomes some problems existing in the traditional homogeneous Fenton reaction, and can effectively remove antibiotics in water, which has good application potential. However, the degradation efficiency and mineralization rate of antibiotics in water by ZVI/H2O2 technology alone need to be improved. Therefore, researchers have adopted different strengthening measures to improve the deconta mination efficiency of ZVI/H2O2 technology and its mineralization rate of pollutants. In this paper, the research of antibiotics removal in water by ZVI/H2O2 technology is statistically analyzed. The main strengthening measures of ZVI/H2O2 technology and their effects on the system are summarized. The degradation efficiency, mechanism, advantages and disadvantages of antibiotics in water by different strengthening measures combined with ZVI/H2O2 technology are described and analyzed. Finally, this paper looks forward to the future development of ZVI/H2O2 technology for the degradation of antibiotics in water, and puts forward relevant suggestions for further research work.
1 Introduction
2 Development status of ZVI/H2O2 technology for removing antibiotics in water at home and abroad
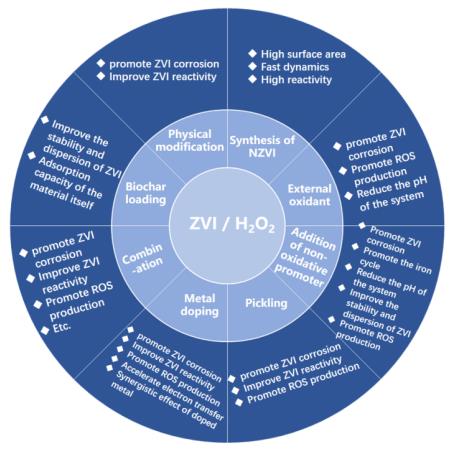
3 The main strengthening measures of ZVI/H2O2 technology and its effect on the system
3.1 Physical modification
3.2 Synthesis of n-ZVI
3.3 Biochar loading
3.4 External oxidant
3.5 Addition of non-oxidative promoter
3.6 Pickling
3.7 Metal doping
3.8 Other
3.9 combination
4 The degradation efficiency and mechanism of antibiotics in water by ZVI/H2O2 technology
5 Conclusion and outlook
Key words: zero-valent iron; H2O2; Fenton-like; antibiotics; strengthening measures
Baizhou Lu , Zhanqiang Fang . Degradation of Antibiotics Using ZVI/H2O2 Fenton-Like Technology[J]. Progress in Chemistry, 2025 , 37(3) : 411 -424 . DOI: 10.7536/PC240509
图1 Publication Status of Relevant Literature on Fenton-like Technologies: (a) Annual Publication Volume from 2014 to 2023; (b) Publication Volume of the Top 10 Countries with the Highest Publication Volume (Data Source: CNKI and Web of ScienceTM)Fig. 1 Publication of relevant papers on Fenton-like technology (2014—2023).(a) Annual volume of papers issued; (b) highest number of papers issued by each country (data from China National Knowledge Infrastructure and Web of scienceTM) |
图2 Clustering Map of Fenton-like Technology Keywords (Data Source: Web of ScienceTM)Fig. 2 Keywords clustering diagram of Fenton-like technology (data from Web of scienceTM).(data from China National Knowledge Infrastructure and Web of scienceTM) |
 . Pan et al.[19] also enhanced the contaminant removal efficiency of the ZVI/H2O2 system by external UV irradiation. The results showed that within 60 minutes, the removal rate of SMT in water by the standalone ZVI/H2O2 system was less than 10%, whereas the removal rate of the UV/ZVI/H2O2 system significantly increased to 99.4%. Additionally, Ambika et al.[47] significantly improved the reactivity of ZVI by ball milling (10 hours) to strip the passivation film on the ZVI surface, thereby enhancing the ability of the ZVI/H2O2 system to simultaneously treat Cr(VI) and phenol in wastewater (Figure 4a). Segura et al.[31] utilized ultrasonic (5 minutes) assisted ZVI/H2O2 Fenton-like technology, which enhanced the reactivity of the ZVI/H2O2 system and greatly improved the mineralization rate of phenol in sewage. Chen et al.[37] adopted microwave (MW) synergistic ZVI/H2O2 Fenton-like technology to treat high-concentration landfill leachate. The results indicated that at 14 minutes, compared with the ZVI/H2O2 system, the COD removal rate of the MW-ZVI/H2O2 system significantly increased from 17.90% to 76.38% (Figure 4b). Although external auxiliary means can achieve effective removal and mineralization of pollutants, these methods often rely on excessively low initial pH and are costly, limiting the large-scale application of physically assisted ZVI/H2O2 Fenton-like technology.
. Pan et al.[19] also enhanced the contaminant removal efficiency of the ZVI/H2O2 system by external UV irradiation. The results showed that within 60 minutes, the removal rate of SMT in water by the standalone ZVI/H2O2 system was less than 10%, whereas the removal rate of the UV/ZVI/H2O2 system significantly increased to 99.4%. Additionally, Ambika et al.[47] significantly improved the reactivity of ZVI by ball milling (10 hours) to strip the passivation film on the ZVI surface, thereby enhancing the ability of the ZVI/H2O2 system to simultaneously treat Cr(VI) and phenol in wastewater (Figure 4a). Segura et al.[31] utilized ultrasonic (5 minutes) assisted ZVI/H2O2 Fenton-like technology, which enhanced the reactivity of the ZVI/H2O2 system and greatly improved the mineralization rate of phenol in sewage. Chen et al.[37] adopted microwave (MW) synergistic ZVI/H2O2 Fenton-like technology to treat high-concentration landfill leachate. The results indicated that at 14 minutes, compared with the ZVI/H2O2 system, the COD removal rate of the MW-ZVI/H2O2 system significantly increased from 17.90% to 76.38% (Figure 4b). Although external auxiliary means can achieve effective removal and mineralization of pollutants, these methods often rely on excessively low initial pH and are costly, limiting the large-scale application of physically assisted ZVI/H2O2 Fenton-like technology.图4 (a) Mechanism of Cr(VI) and Phenol Simultaneous Removal by Ball Milling mZVI/H2O2[47]; (b) Reaction Mechanism of MW-ZVI/H2O2 System for Treating High-Concentration Landfill Leachate[37]Fig. 4 (a) Mechanism of simultaneous removal of Cr(VI) and phenol by ball milled mZVI/H2O2 [47]; (b) mechanism of MW-ZVI/H2O2 system treating high concentration landfill leachate[37] |
 ) in the same water sample were also efficiently degraded (degradation rates of 77%~100%). Similarly, Li et al.[66] enhanced the corrosion rate of iron in the ZVI/H2O2 system and the decomposition rate of H2O2 by adding PS, significantly improving the removal efficiency of erythromycin (ERY) in wastewater. Within 90 minutes, the system completely degraded ERY (Figure 7). Although adding external oxidants can significantly enhance the pollutant removal efficiency of the ZVI/H2O2 Fenton-like system, it may cause secondary pollution from sulfate radicals, posing a threat to the ecological environment and human health.
) in the same water sample were also efficiently degraded (degradation rates of 77%~100%). Similarly, Li et al.[66] enhanced the corrosion rate of iron in the ZVI/H2O2 system and the decomposition rate of H2O2 by adding PS, significantly improving the removal efficiency of erythromycin (ERY) in wastewater. Within 90 minutes, the system completely degraded ERY (Figure 7). Although adding external oxidants can significantly enhance the pollutant removal efficiency of the ZVI/H2O2 Fenton-like system, it may cause secondary pollution from sulfate radicals, posing a threat to the ecological environment and human health.图8 (a) Reaction Mechanism of NOR Removal in Water by Guava Leaf Extract/ZVI/H2O2 System[70]; (b) Reaction Mechanism of NOR in Water by Eucalyptus Leaf Extract/ZVI/H2O2 System[71]; (c) Reaction Mechanism of Landfill Leachate Treatment by Moringa Seed Extract/ZVI/H2O2 System[74]; (d) Reaction Mechanism of Copper Ion Removal from Wastewater by Bone Glue/ZVI/H2O2 System[75]Fig. 8 (a) Mechanism of guava leaf extract/ZVI/H2O2 system to remove NOR in water; (b) mechanism of eucalyptus leaf extract/ZVI/H2O2 system to remove NOR in water; (c) mechanism of landfill leachate treatment by Moringa oleifera seed extract/ZVI/H2O2 system; (d) mechanism of removing copper ions in wastewater by bone glue/ZVI/H2O2 system |
图9 (a) Schematic Diagram of the Mechanism for Simultaneous Removal of Aniline, Hexavalent Chromium, and Antimony by ZVI/H2O2 Process[84]; (b) Reaction Mechanism of Acid Washing-Zero Valent Iron Fenton-like System[35]Fig. 9 (a) Mechanism of simultaneous removal of aniline, Cr(Ⅵ) and antimony by ZVI/H2O2 process[84]; (b) mechanism of pickling-ZVI Fenton-like system[35] |
图10 (a) Reaction Mechanism of Hydrothermal Conversion of Cathode Materials of Lithium-Ion Batteries into MnO2/ZVI Composites[87]; (b) Schematic Diagram of the Reaction Mechanism of Fe-Cu Bimetallic Materials[88]Fig. 10 (a) Hydrothermal conversion of Li-ion battery cathode materials into MnO2/ZVI composites: mechanis[87]; (b) mechanism of Fe-Cu bimetallic material[88] |
图12 (a) Reaction Mechanism of UV/pre-Fe0/H2O2 Process for Antibiotic Degradation[19]; (b) Reaction Mechanism of MoS2-Enhanced ZVI-EF Process for SMT Degradation[94]; (c) Reaction Mechanism of S-ZVI/H2O2 Process for Pollutant Degradation[95]; (d) Reaction Mechanism of UV/nZVI/H2O2 for CIP Removal in Water[36]; (e) Reaction Mechanism of Photocatalytic-nZVI/H2O2 Process for SDZ Removal in Water[58]Fig. 12 (a) Mechanism of antibiotics degradation by UV/pre-Fe0/H2O2 process[19]; (b) mechanism of MoS2 enhanced ZVI-EF process for SMT degradation[94]; (c) mechanism of pollutants degradation by S-ZVI/H2O2 process[95]; (d) mechanism of CIP removal in water by UV/nZVI/H2O2 [36]; (e) mechanism of SDZ removal in water by photocatalysis-nZVI/H2O2 process[58] |
表1 不同强化措施去除水中抗生素的修复效能及其作用机理Table 1 Remediation efficiency and mechanism of different strengthening measures to remove antibiotics in water |
| Strengthening measures | Antibiotic | Initial concentrationof antibiotics | Catalyst dosage | H2O2 dosage | InitialpH | Reactiontime | Remediation efficiency | Main mechanism | Ref | |
|---|---|---|---|---|---|---|---|---|---|---|
| Physical modification | Place ZVI in 200 mT magnetic field for 2 min | SMT | 400 mg/L | 0.3 mmol/L | 0.3 mmol/L | 7.3 | 60 min | About 5% higher than ZVI/H2O2 system | Promote ZVI corrosion; Promote ROS production; Fenton-like oxidation | 19 |
| 6 W UV | SMT | 400 mg/L | 0.3 mmol/L | 0.3 mmol/L | 7.3 | 60 min | 99.4% | Direct Photolysis; Fenton-like oxidation | 19 | |
| Weak magnetic field | SMX | 20 μmol/L | 56 mg/L | 0.1 mmol/L | 3~4 | 15 min | Almost completely removed | Promote ZVI corrosion; Fenton-like oxidation | 49 | |
| Synthesis of nZVI | Borohydride reduction method | NOR | 100 mg/L | 100 mg/L | 20 mmol/L | 3~4 | 40 min | 95% | Adsorption; Complexation; Fenton-like oxidation | 54 |
| Borohydride reduction method | AMX | 50 mg/L | 500 mg/L | 6.6 mmol/L | 3 | 21 min | 86.5% | Adsorption; Fenton-like oxidation | 55 | |
| Liquid phase reduction method | MNZ | 80 mg/L | 500 mg/L | 3.24 mmol/L | 3.02 | 5 min | 100% | Reduction followed by oxidation | 56 | |
| Green Synthesis of Tragacanth gum | AMX,CIP | —— | 765 mg/L | 20 mmol/L | 3.5 | 60 min | AMX 90% CIP 51% | Complexation; Fenton-like oxidation | 57 | |
| Green Synthesis of Black tea extract | SDZ | 50 μM | 1.92 mmol/L | 1.92 mmol/L | 4 | 60 min | 90% | Adsorption; Reduction; Fenton-like oxidation | 58 | |
| Green Synthesis of oak leaves extract | AMX | 10 mg/L | 0.024 mmol/L | 0.31 mmol/L | 3 | 15 min | 100% | Biodegradation; Reduction; Fenton-like oxidation | 59 | |
| Green Synthesis of Tea polyphenols | LCM | 20 mg/L | 100 mg/L | 1 mmol/L | 5.8 | 90 min | 94% | Chelation; Fenton-ike oxidation | 60 | |
| Biochar loading | Biochar was produced by the pyrolysis of miscanthus floridulus | CIP | 100 mg/L | 400 mg/L | 20 mmol/L | 3~4 | 40 min | 70% | Adsorption; Fenton-like oxidation | 63 |
| Biochar was produced via pyrolysis of bamboo sawdust | ONZ | 100 mg/L | 300 mg/L | 12 mmol/L | 3 | 12 min | 80.10% | Adsorption; Fenton-ike oxidation | 64 | |
| External oxidant | PS | 9 different PPCPs | 50 mg/L | 8 mmol/L | 2 mmol/L | 7 | 30 min | 77%~100% | Reduces pH and acidifies contaminants; Fenton-like oxidation | 65 |
| PS | ERY | 1 mg/L | 22.4 mg/L | 0.09 mmol/L | 6 | 90 min | 100% | Promote ZVI corrosion; Fenton-like oxidation | 66 | |
| Addition of non-oxidative promoter | Tea polyphenols | LCM | 20 mg/L | 500 mg/L | 1 mmol/L | 5.8 | 90 min | 97% | Chelation; Tea polyphenols chelated first and then reduced iron ions; Fenton-like oxidation | 68 |
| Oxalic acid; Hydroxyla mine hydrochloride; Ascorbic acid | LCM | 20 mg/L | 500 mg/L | 1 mmol/L | 5.8 | 90 min | 100% | Introduction of acidic groups; Promoters chelated first and then reduced iron ions; Fenton-like oxidation | 70 | |
| Green tea extract (GT), Black tea extract (BT), Yellow tea extract (YT), Dark tea extract (DT), White tea extract (WT), Oolong tea extract (OT) | NOR | 10 mg/L | 500 mg/L | 1 mmol/L | 7 | 120 min | GT 98.84%, BT 87.92%, YT 80.11%, DT 79.16%, WT 68.03%, OT 63.94% | Promote the iron cycle; Fenton-like oxidation | 69 | |
| Metal doping | Microscale Fe/Cu bimetallic particles’ catalysis | TC | 50 mg/L | 5000 mg/L | 50 mmol/L | 3 | 3 min | 98% | Improve ZVI reactivity; Fenton-like oxidation | 86 |
| Hydrothermal synthesis of MnO2/ZVI composites from Li-ion battery cathodes | SDZ | 20 mg/L | 200 mg/L | 6 mmol/L | 3 | 60 min | 98.60% | Improve ZVI reactivity; Fenton-like oxidation | 87 | |
| Novel Ce-mediated Fe-MIL-101 (Fe/Ce-MIL-101) | NOR | 10 mg/L | 300 mg/L | 20 mmol/L | 7 | 180 min | 100% | Improve ZVI reactivity; Accelerate electron transfer; Fenton-like oxidation | 89 | |
| Others | Metal-organic frameworks (MOFs) derived zero-valent iron embedded in the carbon matrix structure named FMC. | AMX | 20 mg/L | 100 mg/L | 5 mmol/L | 4 | 60 min | 100% | Accelerate electron transfer; Fenton-like oxidation | 91 |
| Two-steps method including reducing roasting and magnetic separation was implemented by reutilizing red mud as iron sources and activate carbon as reducing agent. | SDZ | 20 mg/L | 100 mg/L | 1 mmol/L | 3 | 10 min | 100% | Adsorption; Fenton-like oxidation | 92 | |
| Reduce graphene oxide (rGO) to support nZVI to synthesize rGO-nZVI nanohybrid. | 12 different PPCPs | 0.2 mg/L | 407 mg/L | 10 mmol/L | 3 | 10 min | 95%~99% | Adsorption; Fenton-like oxidation | 93 | |
| Combination | UV/pre-magnetized ZVI | SMT, OTC, TC | SMT 400 mg/L, OTC 800 mg/L, TC 800 mg/L | 0.3 mmol/L | 0.3 mmol/L | 7.3 | 30 min | 100% | Promote ZVI corrosion; Promote ROS; Direct Photolysis; Fenton-like oxidation | 19 |
| MoS2 as highly efficient co-catalyst enhancing the performance of ZVI based electro-Fenton process | SMT | 10 mg/L | 224 mg/L | —— | 4 | 10 min | 100% | MoS2 co-catalysis; Promote the iron cycle; Fenton--ike oxidation | 94 | |
| Mechanochemically Sulfidated ZVI | TC | 0.2 mM | 120 mg/L | 2 mmol/L | 3 | 20s | 100% | Accelerated electron transfer and iron precipitation; Fenton-like oxidation | 98 | |
| UV/nZVI/H2O2 | CIP | 10 mg/L | 5 mmol/L | 1 mmol/L | 6.5~7.5 | 25 min | 100% | Improve ZVI reactivity; Fenton-like oxidation | 36 | |
| Green synthesis of nZVI from Black tea extracts with synergistic photocatalysis | SDZ | 50 μmol/L | 1.92 mmol/L | 1.92 mmol/L | 4 | 5 min | 100% | Direct Photolysis; Adsorption; Reduction; Fenton-like oxidation | 58 | |
| The nanocomposites Ce0/Fe0-reduced graphene oxide (Ce0/Fe0-RGO) | SMT | 20 mg/L | 500 mg/L | 8 mmol/L | 7 | 30 min | 99% | Adsorption; Fenton-like oxidation | 97 | |
| [1] |
|
| [2] |
|
| [3] |
(孙玉杰, 杨淑慎, 张琪, 武培, 魏娟. 山东化工, 2017, 46(16): 9.).
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(聂晓静, 大连理工大学硕士论文, 2017.).
|
| [23] |
(韩跃飞, 华东理工大学硕士论文, 2019.
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
(陈俊毅, 易云强, 方战强, 晏晓敏, 郑刘春. 华南师范大学学报(自然科学版), 2019, 51(1): 49.).
|
| [28] |
|
| [29] |
|
| [30] |
(万玲, 洪军, 刘保锋, 苏趋. 中国测试, 2015, 41(10): 44.).
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
(王炫栋, 伯绍毅, 季文浩, 王林拓, 李小忠, 陈寒松. 中国资源综合利用, 2020, 38(6): 4.).
|
| [47] |
|
| [48] |
(黄挺, 张光明, 张楠, 种珊, 刘毓璨, 朱佳. 环境工程学报, 2017, 11(11): 5892.).
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
(易云强, 方战强. 华南师范大学学报(自然科学版), 2018, 50(1): 28.).
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
(柴凡凡, 李克艳, 郭新闻. 应用化学, 2016, 33(02): 133.).
|
| [68] |
|
| [69] |
(方战强, 卢柏舟, 冼靖怡. 中国专利. CN111747504A. 2020.).
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
(邹亚辰, 贾小宁, 冉浪, 周林成, 赵泉林, 叶正芳. 化学反应工程与工艺, 2021, 37(2): 167.).
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
(王麒, 薛罡, 钱雅洁, 刘振鸿. 工业水处理, 2019, 39(9): 87.).
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
(杨波, 张永丽. 化学学报, 2019, 77(10): 1017.).
|
| [91] |
|
| [92] |
(李瑜辉, 谢武明, 吕文东, 黄子峻, 卞求实. 环境化学, 2022, 41(2): 707.).
|
| [93] |
|
| [94] |
|
| [95] |
(黄丹维, 何佳, 谷亚威, 何锋. 化学学报, 2017, 75(09): 866.).
|
| [96] |
|
| [97] |
|
| [98] |
|
/
| 〈 |
|
〉 |