

2D Perovskites Based on Halogen-Substituted Spacer Cations in Solar Cells
Received date: 2024-07-04
Revised date: 2024-10-22
Online published: 2025-03-10
Supported by
National Natural Science Foundation of China(52102196)
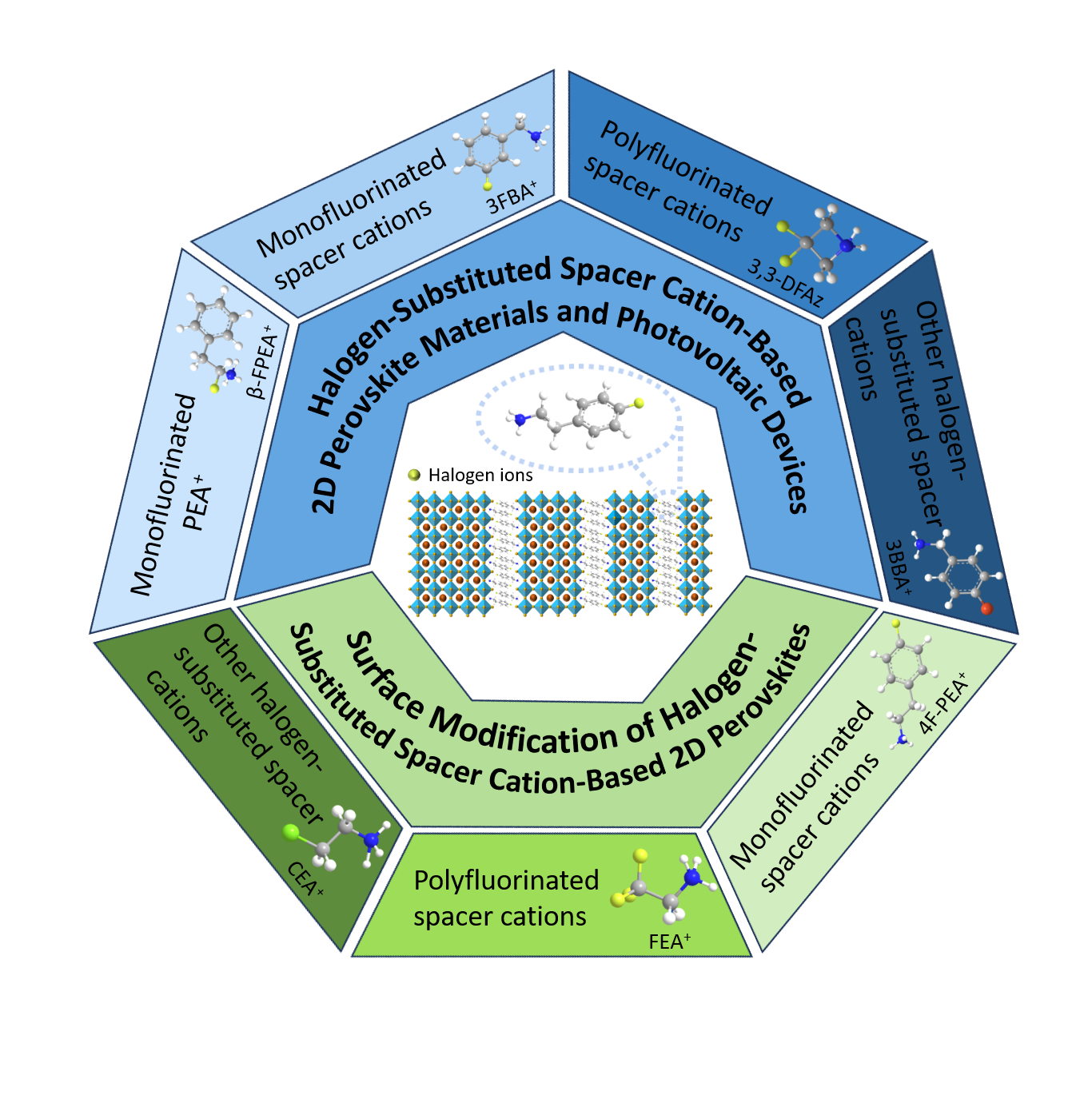
Two-dimensional (2D) perovskite materials have been receiving considerable attention owing to their high stability. Despite this,there is still significant potential for improving their power conversion efficiency. Designing effective spacer cations is one of the crucial methods to improve the photoelectric performance of 2D perovskite solar cells. Among the various strategies,halogen substitution has emerged as a particularly effective approach,which can fine-tune the stability and optical properties of the perovskite crystal structure,leading to notable improvements in photoelectric conversion efficiency as well as long-term stability. In recent years,there has been significant and notable progress of two-dimensional (2D) perovskites based on various halogen-substituted spacer cations in the preparation of high-performance perovskite solar cells. This paper initially provides a comprehensive overview of the development status of 2D perovskite materials and devices that employ different spacer cations. Following this,the focus shifts to an in-depth review of the advancements made in the fabrication of 2D perovskite solar cells (PSCs) and the surface modification of three-dimensional (3D) perovskites,specifically emphasizing the role of spacer cations that have been singly or multiply substituted with halogens such as fluorine,chlorine,and bromine. Finally,we present a concise discussion on the current challenges faced in this field and offer insights into the potential future directions for further research and development.
Contents
1 Introduction
2 2D perovskite materials and devices with different spacer cations
3 Characteristics of halogen-substituted spacer cation-based 2D perovskites and their applications in photovoltaic devices
3.1 Research on halogen-substituted spacer cation-based 2D perovskites and photovoltaic devices
3.2 Research on halogen-substituted 2D perovskite surface modification of 3D perovskites
4 Conclusion and future perspectives on halogen-substituted 2D perovskites
Chaoyang Wu , Chao Wang , Feifan Chen , Xinhe Dong , Haiying Zheng . 2D Perovskites Based on Halogen-Substituted Spacer Cations in Solar Cells[J]. Progress in Chemistry, 2025 , 37(4) : 575 -592 . DOI: 10.7536/PC240618
表1 基于单氟取代的苯乙胺2D PSCs性能总结Table 1 Summary of the performance for the 2D PSCs based on monofluorinated phenylethylamine |
| Spacer cations | Structure of PSCs | n | PCE (%) | Stability | Ref |
|---|---|---|---|---|---|
| mF1PEA+ | ITO/PEDOT:PSS/2D perovskite/PCBM/bathophenanthroline/Al | 4 | >10% | Unencapsulated,RH≈45%,30 days,maintaining approximately 60% of the initial PCE | 65 |
| 4FPEA+ | ITO/PTAA/2D perovskite/PCBM/PEI/Ag | 5 | 17.3% | Unencapsulated,RH=55%~65%,500 h in the air,maintaining more than 93% of the initial PCE | 56 |
| F-PEA+ | ITO/PTAA/2D perovskite/ PC61BM/PEI/Ag | 4 | 18.10% | Unencapsulated,RH=40%~50%,in the air and 80 ℃ under nitrogen,4 weeks,maintaining 90% and more than 80% of the initial PCE,respectively | 66 |
| F-PEA+ | FTO/TiO2/perovskite/ Spiro-OMeTAD/Au | 9 | 16.15% | Unencapsulated,RH=30%~70%,in the air,2112 h,maintaining 95% of the initial PCE | 67 |
| 4FPEA+ | ITO/PTAA/2D perovskites/PCBM/BCP/Ag | 5 | 21.07%(20% certified) | Unencapsulated,85 ℃ under nitrogen,1500 h,maintaining 97% of the initial PCE | 68 |
| β-FPEA+ | ITO/PEDOT:PSS or PTAA/2D RP perovskite/PC61BM/BCP/Ag | 5 | 17.04%(PEDOT:PSS)/19.11%(PTAA) | Unencapsulated,70 ℃ under nitrogen,720 h and RH=35±5%,dark environment,500 h,maintaining 90% and 86% of the initial PCE,respectively | 69 |
| F-PEA+ | FTO/NiOx/perovskite/PCBM/BCP/Ag | 4 | 15.2% | Unencapsulated,RH=30±5%,dark environment,1510 h and RH=85%,530 h,maintaining 83% and 67% of the initial PCE,respectively | 70 |
图2 (a) 苯乙胺及其氟化衍生物的分子结构[65];(b) (4FPEA)2MA4Pb5I16钙钛矿晶体的(111)和(202)取向示意图[56];(c) 准2D钙钛矿的有序和随机n相分布[66];(d) MA和FA基2D钙钛矿的晶体取向和相分布示意图(MA薄膜垂直取向;FA薄膜倾斜取向)[68];(e) 薄膜降解过程示意图[69]Fig.2 (a) Molecular structures of PEA and its fluorinated derivatives[65]. Copyright 2019,Springer Nature;(b) Schematic illustration of the (111) and (202) orientations of (4FPEA)2MA4Pb5I16 perovskite crystal[56]. Copyright 2019,Wiley-Blackwell;(c) orderly and random n phase distributions for Q-2D perovskite[66]. Copyright 2021,Wiley-VCH Verlag;(d) schematic diagrams of the crystal orientation and phase distribution of MA and FA-based 2D perovskites (MA film: graded vertical alignment;FA film: oblique alignment)[68]. Copyright 2022,Wiley-Blackwell;(e) schematic diagram of the film degradation process[69]. Copyright 2023,Wiley-Blackwell |
表2 基于单氟取代的其他间隔阳离子2D PSCs性能总结Table 2 Summary of the performance for 2D PSCs based on other monofluorinated spacer cations |
| Spacer cations | Structure of PSCs | n | PCE (%) | Stability | Ref |
|---|---|---|---|---|---|
| FSAI | - | 1,2 | - | RH=60%,in the air,15 days. PXRD peaks remain unchanged | 69 |
| p-FPhFA+ | ITO/PEDOT:PSS/2D perovskite/ PCBM/BCP/Ag | 5 | 17.37% | Unencapsulated,under nitrogen 3000 h and continuous light 200 h,maintaining 99% and 77% of the initial PCE,respectively | 62 |
| 3FBEA+ | ITO/PTAA/3FBAI-based PVK/ PC61BM/BCP/Ag | 5 | 20.12% | Unencapsulated,RH=35%,dark environment,792 h and 55 ℃,672 h,maintaining 91.5% and 93% of the initial PCE,respectively | 72 |
| F-BZA+ | ITO/PTAA/Q-2D perovskite/ PCBM/PEI/Ag | 4 | 16.82% | Unencapsulated,25 ℃,RH≈40%,35 days,maintaining more than 80% of the initial PCE | 73 |
| FPA+ | FTO/TiO2/perovskite/ Spiro-OMeTAD/MoO3/Ag | 5 | 15.18% | RH=25%~30% and 80 ℃ under nitrogen. Black phase stability is maintained for more than 168 h and 96 h,respectively | 74 |
| 3FBA+ | ITO/PEDOT:PSS/(3FBA)2(MA)6Pb7I22/ DiMe-PTCDI/Cr2O3/Au | 7 | 18.5% | Unencapsulated,in nitrogen in the dark or 21±2 ℃,RH=60%±10%, 43 days,all maintaining 80% of the initial PCE | 75 |
| pFBA+ | ITO/PTAA/Q-2D Perovskite/ PC61BM/BCP/Ag | 5 | 17.12% | Unencapsulated,25 ℃,RH=60%±5%,600 h,maintaining 83.13% of the initial PCE | 71 |
图3 (a) 苯甲脒和对氟苯甲脒的化学结构和分子偶极矩及对氟苯甲脒基2D RP钙钛矿(n=5)的结构示意图[62];(b) 苄基铵及氟化苄基铵Q-2D钙钛矿择优晶体取向示意图[73];(c) 苯胺及4-氟苯胺的静电势图和分子偶极矩[74];(d) 钙钛矿结晶机理示意图[75];(e) 四种阳离子的静电表面势和PSCs中的能级排列[71]Fig.3 (a) Chemical structures and molecular dipole moments of PhFA and p-FPhFA,and schematic structure of 2D RP perovskite p-FPhFA-Pb (n=5)[62]. Copyright 2019,American Chemical Society;(b) schematic diagrams of the preferred crystal orientation for BZA- and F-BZA-based Q-2D perovskites[73]. Copyright 2019,American Chemical Society;(c) ESP diagram and molecule dipole moments of PA+ and FPA+[74]. Copyright 2022,Wiley-Blackwell;(d) illustration of the perovskite crystallization mechanism[75]. Copyright 2023,Wiley-Blackwell;(e) electrostatic surface potential of four cations and energy-level alignments in PSCs[71]. Copyright 2022,American Chemical Society |
表3 基于多氟取代的间隔阳离子2D PSCs性能总结Table 3 Summary of the performance for 2D PSCs based on polyfluorinated spacer cation |
| Spacer cations | Structure of PSCs | n | PCE (%) | Stability | Ref |
|---|---|---|---|---|---|
| TFBDA+ | ITO/SnO2/2D DJ perovskite/ Spiro-OMeTAD/Au | 10 | 15.24% | Unencapsulated,RH=40%~70%,in the air,1300 h and annealing at 80 ℃,100 h,maintaining 93% and more than 80% of the initial PCE,respectively | 76 |
| 3FBEA+ | ITO/PEDOT:PSS/DJ perovskite/ PCBM/BCP/Ag | 4 | 16.62% | Unencapsulated,room temperature,dark environment under nitrogen,1839 h and continuous light (100 mW·cm-2),186 h and 80 ℃ dark environment under nitrogen,350 h,maintaining 93%,93% and more than 94% of the initial PCE,respectively | 77 |
| F3EA+-BA+ | ITO/PEDOT:PSS/perovskite/ PC61BM/BCP/Ag | 4 | 12.51% | Under nitrogen,216 h,maintaining more than 80% of the initial PCE | 78 |
| 5FPTM++PTMA+ | ITO/PEDOT:PSS/perovskite/ PC61BM/BCP/Ag | 5 | 18.56% | Unencapsulated,70 ℃ under nitrogen,936 h and continuous light (100 mW·cm-2) 873 h,maintaining 92% and 93% of the initial PCE,respectively | 79 |
| 3,3-DFAz | ITO/PTAA/2D RP perovskite/ PCBM/BCP/Ag | 4 | 19.85% | Unencapsulated,60 ℃ under nitrogen,dark environment,1100 h and RH=35%±5%,900 h,maintaining more than 80% and 90% of the initial PCE,respectively | 80 |
图4 (a) (4F-PhDMA)PbI4钙钛矿的晶体结构[77];(b) F3EAI对层状2D钙钛矿[(BA)1-x(F3EA)x]2(MA)3Pb4I13材料中束缚激子的影响示意图[78];(c) PTMA-Pb和5F/PTMA-Pb钙钛矿薄膜的成核以及结晶机制示意图[79];(d) 基于不同钙钛矿材料的PSCs能级图[80];(e) Q-2D钙PSCs的能级图[63];(f) 0.1FBA和0.1CBA钙钛矿薄膜的氟和氯元素,卤素和FA阳离子之间的氢键和卤素和金属离子之间离子键示意图及引入CBA和FBA后促进准2D钙钛矿性能提高的过程示意图[82]Fig.4 (a) Crystal structure of the (4F-PhDMA)PbI4 perovskite[77]. Copyright 2021,American Chemical Society;(b) schematic representation of the effect of F3EAI on bounded excitons in layered 2D perovskite [(BA)1-x(F3EA)x]2(MA)3Pb4I13 materials[78]. Copyright 2019,Wiley-VCH Verlag;(c) schematic for the proposed nucleation and crystallization mechanism of PTMA-Pb and 5F/PTMA-Pb perovskite films[79]. Copyright 2024,John Wiley and Sons Ltd;(d) energy level diagrams of the materials used in PSCs[80]. Copyright 2024,John Wiley and Sons Ltd;(e) energy level diagram of the quasi-2D perovskite solar cell[63]. Copyright 2018,Wiley-Blackwell;(f) fluorine and chlorine element on the 0.1FBA and 0.1CBA perovskite films,schematic of the hydrogen bonds between halogens and FA cations and the ionic bonds between the halogens and metal ions and schematic of the process of promoting the improvement of quasi-2D perovskite performance after the incorporation of CBA and FBA[82]. Copyright 2020,Royal Society of Chemistry |
表4 基于单氟取代的间隔阳离子2D钙钛矿用于表面修饰的器件性能总结Table 4 Summary of the performance for PSCs with surface modification using monofluorinated spacer cations |
| Spacer cations | Structure of PSCs | PCE (%) | Stability | Ref |
|---|---|---|---|---|
| 4FPEA+ | ITO/SnO2/perovskite/ Spiro-OMeTAD/Au | 20.53% | Unencapsulated,18~23 ℃,RH=20%~30%,1000 h,maintaining 86% of the initial PCE | 85 |
| 4FPEA+ | ITO/PEDOT:PSS/perovskite/ PC61BM/BCP/Ag | 17.51% | Unencapsulated,under nitrogen,1200 h,maintaining 90% of the initial PCE | 86 |
| 4FPEA+ | FTO/c-TiO2/m-TiO2/perovskite/ spiro-OMeTAD/Au | 20.5% | Unencapsulated,room temperature,RH=85%,about 1000 h and 80 ℃,700 h,all maintaining more than 90% of the initial PCE | 87 |
| FPEA+ | ITO/P3CT-MA/perovskite/PCBM/ BCP/Ag | 22.53% | 85 ℃,RH=40%±5%,500 h,maintaining approximately 90% of the initial PCE | 88 |
| 4FPEA+ | FTO/c-TiO2/m-TiO2/perovskite/ spiro-OMeTAD/Au | 21.79% | Unencapsulated,RH=85%,1080 h and 85 ℃,RH=85%,500 h,maintaining 86% and 80% of the initial PCE,respectively | 89 |
| 4FPEA+ | FTO/c-TiO2/m-TiO2/perovskite/ spiro-OMeTAD/Au | 23.18% | Unencapsulated,85 ℃,RH=85%,dark environment,300 h,maintaining 83% of the initial PCE | 90 |
图5 (a) (FPEA)2PbI4覆盖层在抵抗湿气侵入的关键作用示意图[85];(b) FPEAAc和PEA旋涂到3D钙钛矿表面上退火前后过程[88];(c) 在3D钙钛矿与碳界面构建2D钙钛矿的示意图[93];(d) 基于单阳离子1%-PEA和混合阳离子0.75%-F5PEA的2D钙钛矿钝化剂修饰的3D钙钛矿薄膜C-AFM图[94];(e) 基于FPEAI和5BzAI的2D钙钛矿表面静电势[95]Fig.5 (a) Schematic illustration depicting the key role of the (FPEA)2PbI4 capping layer in resisting moisture ingress[85]. Copyright 2021,Wiley-Blackwell;(b) FPEAAc and PEA spin-coated onto 3D perovskite surfaces during and after annealing[88]. Copyright 2023,American Chemical Society;(c) scheme for constructing 2D perovskite at the interface between 3D perovskite and carbon[93]. Copyright 2021,Wiley-VCH Verlag;(d) C-AFM of 3D perovskite thin films based on mono-cation 1%-PEA and mixed-cation 0.75%-F5PEA 2D perovskite passivation agent[94]. Copyright 2020,Wiley-Blackwell;(e) electrostatic potential at the surface of 2D perovskites containing FPEAI and 5BzAI cations[95]. Copyright 2020,Wiley-VCH Verlag |
表5 基于多氟取代的间隔阳离子2D钙钛矿用于表面修饰的器件性能总结Table 5 Summary of the performance for PSCs with surface modification using polyfluorinated spacer cations |
| Spacer cations | Structure of PSCs | PCE (%) | Stability | Ref |
|---|---|---|---|---|
| F3EA+ | FTO/c-TiO2/m-TiO2/perovskite/ spiro-OMeTAD/Au | 19.24% | Unencapsulated,RH=50%±5%,aging for 60 days,maintaining 88% of the initial PCE | 91 |
| F5PEA+ | FTO/c-TiO2/m-TiO2/perovskite/ spiro-OMeTAD/Au | >22% | Unencapsulated,RH=40%,sun irradiation for 1000 h,main-taining 90% of the initial PCE | 92 |
| F5PEA+ | Perovskite infiltrates into TiO2/ ZrO2/carbon three-layer mesoporous scaffolds | 17.47% | Unencapsulated,25 ℃,RH=55%~70%,in the air,1000 h,maintaining 95% of the initial PCE | 93 |
| F5PEA+-PEA+ | ITO/PTAA/perovskite/PCB/Ag | 21.10% | Unencapsulated,RH=45%~60%,720 h and 20 ℃ under nitrogen (Resistive load and 0.77 sunlight) 324 h,maintaining more than 83% and 89.1% of the initial PCE,respectively | 94 |
| 5FBzA+ | FTO/TiO2/perovskites/ spiro-OMeTAD/Au | 21.65% | In argon,1100 h of sunlight,maintaining 86% of the initial PCE | 95 |
| CF3PEA+ | ITO/SnO2/perovskite/ spiro-OMeTAD/Au | 21.05% | Unencapsulated,RH=70%~80%,in the air,528 h,maintaining 98% of the initial PCE | 96 |
| m(p)-CF3PEA+ | ITO/SnO2/perovskite/ spiro-OMeTAD/Au | 22.4% | Unencapsulated,25 ℃,RH=65%,385 h,maintaining 85% of the initial PCE | 97 |
| FxPEA+ (x=1,2,3,5) | FTO/SnO2/perovskite/ spiro-OMeTAD/Ag | 22.74% | Unencapsulated,RH=60%±5%,300 h,maintaining 95% of the initial PCE | 98 |
表6 基于其他卤素取代的间隔阳离子2D钙钛矿用于表面修饰的器件性能总结Table 6 Summary of the performance for PSCs with surface modification using other halogenated spacer cations |
| Spacer cations | Structure of PSCs | PCE (%) | Stability | Ref |
|---|---|---|---|---|
| 3BBA+ | ITO/PTAA/perovskite/PC61BM/Cr/Au | 18.20% | Unencapsulated,RH≈40%,2400 h,maintaining more than 82% of the initial PCE. After soaking in water for 60 seconds,the photovoltaic parameters are almost unchanged | 63 |
| CFFA+ | ITO/PEDOT:PSS/DJ perovskite/ Spiro-OMeTAD/Au | 14.78% | Unencapsulated,25 ℃,RH=35%,2000 h,maintaining 80% of the initial PCE | 81 |
| HEA++CBA+ | FTO/c-TiO2/m-TiO2/perovskite/ Spiro-MeOTAD/Au | 18.75% | Unencapsulated,85 ℃,200 h and RH= 45%±5%,1500 h,maintaining 81% and 90% of the initial PCE,respectively | 82 |
| 3Br-BA+ | ITO/C-TiO2/Meso-TiO2/2D perovskite/PC61BM/Cr/Au | 13.21% | - | 83 |
| BBA+ | ITO/c-TiO2/m-TiO2/perovskite/ Spiro-OMeTAD/Au | 21.13% | Unencapsulated,RH=45%±5%,1000 h and RH=15%±5%,3096 h and RH=75%±5%,1800 h,maintaining more than 90%,97% and 80% of the initial PCE,respectively | 99 |
| m-BrPEA+ | FTO/cp-TiO2/mp-TiO2/SnO2/PVK/ Spiro-OMeTAD/Au | 23.42% | Unencapsulated,20~25 ℃,RH>20%,200 days,maintaining 98% of the initial PCE | 100 |
| Cl4Tm+ | ITO/SnO2/Perovskite/PTAA/Au | 24.6% | Unencapsulated,65 ℃,2220 h in nitrogen or 507 h of continuous lighting aging in nitrogen,maintaining 80% of the initial PCE | 101 |
| CBA+ | ITO/SnO2/Perovskite/ Spiro-OMeTAD/Au | 21.03% | Unencapsulated,45 ℃,continuous sunlight irradiation while tracking the maximum power point stability in nitrogen for over 1000 h,maintaining 90% of the initial PCE | 102 |
图6 (a) BAI、FBAI、CBAI和BBAI表面修饰的表面SEM图像[99];(b) 合成和使用的九种不同卤化PEAI盐的结构[100];(c) 3D钙钛矿层上2D钙钛矿的器件结构示意图及设计共轭铵阳离子的化学结构[101];(d) 原理图: 用有限元建模的f-PSC按比例弯曲的6层f-PSC,在3D-MHP层中具有初始表面裂纹、通道和界面裂纹。有限元模拟计算界面裂纹2-3和3-4的晶界韧性[102]Fig.6 (a) Top-view SEM images of the perovskite films modified by BAI,FBAI,CBAI and BBAI[99]. Copyright 2020,Elsevier BV;(b) structure of nine different halogenated PEAI salts synthesized and used[100]. Copyright 2020,Wiley-VCH Verlag;(c) schematic of device structure used with a 2D perovskite layer atop of 3D perovskite and chemical structures of the designed conjugated ammonium cations[101]. Copyright 2020,American Association for the Advancement of Science;(d) schematic illustrations: bending of f-PSC to scale 6-layer f-PSC setup for finite element modeling with an initial surface crack in 3D-MHP layer and with channel and interfacial cracks. Finite element modeling calculated grain boundary toughness for interfacial cracks 2-3 and 3-4[102]. Copyright 2022,Wiley-Blackwell |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
(唐森林, 高欢, 彭颖, 李明光, 陈润锋, 黄维. 化学进展, 2022, 34(8): 1706.).
|
| [12] |
(杨英, 罗媛, 马书鹏, 朱从潭, 朱刘, 郭学益. 化学进展, 2021, 33(2): 281.).
|
| [13] |
(周颖, 刘雪朋, 张先付, 韩明远, 陈建林, 梁永鹏, 李博桐, 丁勇, 蔡墨朗, 戴松元. 化学进展, 2024, 36(5): 613.).
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
(王蕾, 周勤, 黄禹琼, 张宝, 冯亚青. 化学进展, 2020, 32(1): 119.).
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
(戴松元, 潘旭, 郑海英, 叶加久, 姚建曦, 张兵, 陈海彬, 任英科.CN 106098943, 2016.).
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
/
| 〈 |
|
〉 |