

Application of Polyacrylonitrile in the Electrolytes of Lithium Metal Battery
Received date: 2022-09-15
Revised date: 2023-01-14
Online published: 2023-02-16
Supported by
National Natural Science Foundation of China(21975284)
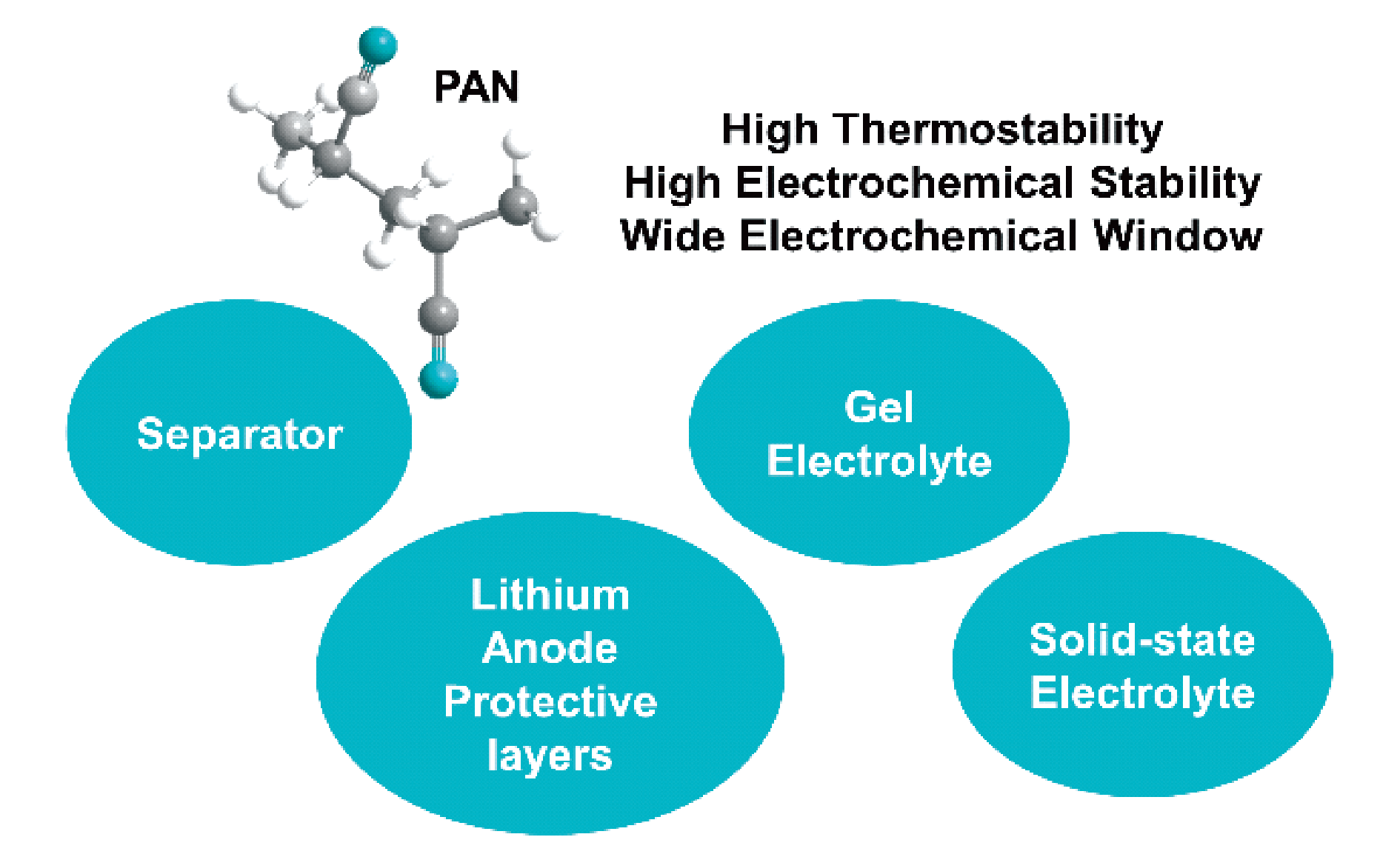
With the rapid development of portable electronic devices, electric vehicles, and smart grids, there is an increasing interest in high-energy-density lithium metal batteries. Uneven Li stripping or deposition on the surface of lithium metal will lead to the growth of lithium dendrites, which can easily pierce the separator and cause the short circuit in the battery. Moreover, the highly reactive lithium metal will continue to react with the electrolyte, resulting in an unstable solid electrolyte. interfacial (SEI) film and irreversible capacity loss. Taking high-energy-density and high safety into account is a key scientific problem that needs to be solved urgently in the development and application of lithium metal batteries. The interaction of strong electron withdrawing group (C≡N) in polyacrylonitrile (PAN) polymer and C=O in carbonate solvent can form a more stable SEI film. As a lithium anode coating, PAN can also inhibit the growth of lithium dendrites. In addition, due to the low lowest unoccupied molecular orbital, high electrochemical stability and wide electrochemical window, PAN can be regard as polymer electrolytes for lithium metal batteries, and matched with a high-voltage cathode to achieve both high energy density and safety. Thus, PAN polymer has significant potential application in electrolytes for lithium metal batteries. This review mainly starts from the different states of electrolytes (liquid, gel, and solid state). Recent research development of PAN polymer as separators and lithium anode protective layers in liquid electrolytes, as well as its application in gel electrolytes and solid-state electrolytes are presented. Finally, the review prospects the development trend of PAN polymer in lithium metal battery electrolytes.
Yu Xiaoyan , Li Meng , Wei Lei , Qiu Jingyi , Cao Gaoping , Wen Yuehua . Application of Polyacrylonitrile in the Electrolytes of Lithium Metal Battery[J]. Progress in Chemistry, 2023 , 35(3) : 390 -406 . DOI: 10.7536/PC220913
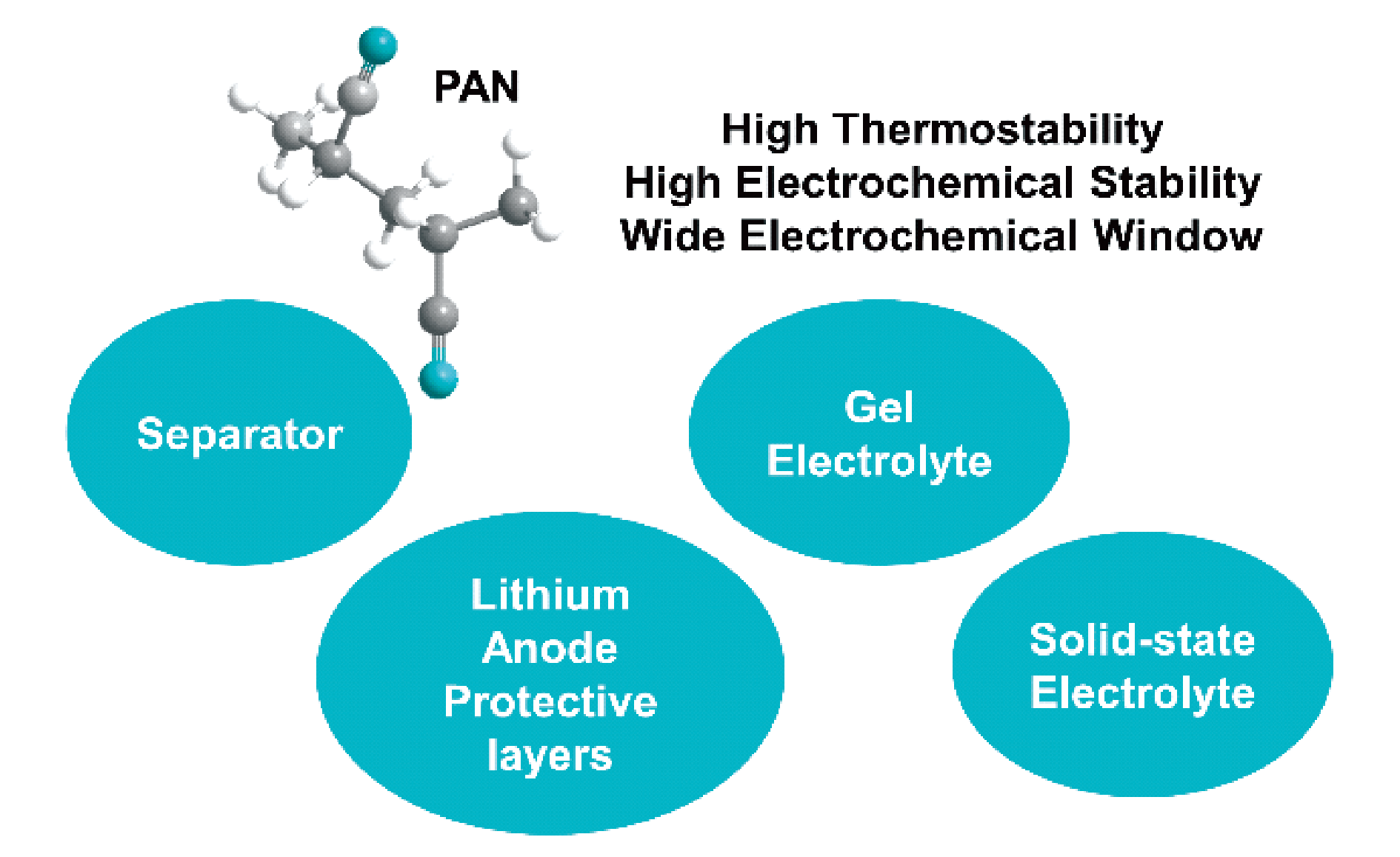
表1 基于PAN聚合物的隔膜的物理性质Table 1 The PAN-based separators and their physical performance |
| Component | Thickness (μm) | σ (mS· cm-1) | Porosity (%) | Electrolyte Uptake (%) | Fracture strength (MPa)/ Elongation (%) | Cathode | ref | |
|---|---|---|---|---|---|---|---|---|
| 1 | PAN | 26 | 0.94 | 62.0 | 300 | 49.6/3.8 | LiFePO4 | 36 |
| 2 | PAN | 24 | 1.06 | 54.7 | 336 | - | LiMn2O4 | 33 |
| 3 | PDA@PAN | 50 | 1.39 | 83.3 | 341 | 13.9/31.3 | LiFePO4 | 37 |
| 4 | PAN/cellulose/ nylon6/PVPK30 | - | - | 55.7 | 225 | 71.24/33.7 | - | 38 |
| 5 | PAN/CA/HAP | 46 | 3.02 | 61 | 268 | 11.8/11.8 | LiFePO4 | 39 |
| 6 | PAN/ZSM-5 | - | 2.16 | 55.5 | 308 | 13/- | LiFePO4 | 40 |
| 7 | PAN/PEO/PAN | 25 | 1.54 | 68 | 650 | - | LiFePO4 | 41 |
| 8 | PAN/DOPO | - | 6.49 | - | 310 | - | LiFePO4 | 42 |
| 9 | PAN/HPTCP | - | 0.95 | 46 | 162 | 40 | NCM622 | 43 |
| 10 | PAN-PEI | 60 | 0.19 | - | - | 19 | S/NCM523 | 44 |
| 11 | PAN-SiO2 | 115 | 1.98 | 85.3 | - | 9.6 | NVP/LiFePO4 | 45 |
图3 (a)在1 mV·s-1扫描速率下的阴极线性扫描曲线;(b)在5 M LiFSI 电解质中、1 mA·cm-2下,不含/含AN添加剂的恒电流锂沉积曲线;(c)代表性Li+溶剂化结构的计算还原电位;(d)Li+-AN、Li+-EC、Li+-DEC和Li+-FSI-对的计算还原电位[51]Fig. 3 (a) Cathodic linear sweep at 1 mV·s-1 scan rate; (b) Galvanostatic Li deposition curves at 1 mA·cm-2 in 5 M LiFSI electrolytes without and with AN additive; (c) Calculated reduction potential of the representative Li+ solvation structures; (d) Calculated reduction potential of Li+-AN, Li+-EC, Li+-DEC, and Li+-FSI- pairs[51]. Copyright 2021, Elsevier |
图4 (a)PAN和EC的静电势图;(b)PAN的C≡N基团和EC的C=O基团之间的偶极-偶极相互作用的示意图;(c)5 mA· cm-2下循环后的裸锂和具有极性聚合物网络涂层的锂片的截面及表面形貌[55];(d)两种聚合物和溶剂分子之间的结合能;(e)在锂锂对称电池中循环5次后的Li/ELPAN和Li/ELPS[56]Fig. 4 (a) Electrostatic potential maps of PAN and EC; (b) Schematic illustration of the dipole-dipole interaction between the C≡N group of PAN and the C=O group of EC; (c) Cross-sectional and surface SEM morphologies of bare Li and Li sheets coated with polar polymer network after cycling under 5 mA·cm-2 [55]. Copyright 2019, Royal Society of Chemistry (d) Binding energy between the two polymers and solvent molecules; (e) Li/ELPAN and Li/ELPS after 5 cycles in a Li-Li symmetric battery[56]. Copyright 2022, Elsevier |
表2 不同填料的PAN聚合物固态电解质Table 2 Solid electrolyte based on blending PAN polymer with different fillers |
| Component | σ(S·cm-1) | Electrochemical window (V) | Cathode | ref | ||
|---|---|---|---|---|---|---|
| 1 | PAN/LiClO4/LLZO nanowires | 1.31×104 (20 ℃) | - | 0.3 | - | 81 |
| 2 | PVA/PAN/LATP/SN/LiTFSI | 1.13×104 (25 ℃) | 5.1 | 0.507 | LiFePO4 | 82 |
| 3 | PAN/LiClO4/LLTO nanotubes | 3.6×104 (RT) | 5 | 0.38 | LiFePO4 | 83 |
| 4 | PAN/LiClO4/LLZTO | 2.2×104 (40 ℃) | 4.9 | 0.3 | LiFePO4 | 84 |
| 5 | PAN/LiClO4/ graphene oxide | 4×104 (30 ℃) | 4.3 | 0.42 | LiFePO4 | 85 |
| 6 | PAN/LiTFSI/SiO2 | 1.8×104 (60 ℃) | 4.8 | 0.47 | LiFePO4 | 86 |
| 7 | PAN/SiO2/LiTFSI/EMIMTFSI | 3.5×104 (20 ℃) | 5.2 | 0.52 | LiFePO4/NCM622 | 22 |
| 8 | SNE@SAG/PAN | 7.45×104 (30 ℃) | 5 | 0.7 | NCM811 | 87 |
图6 (a)用PAN修饰的SCN改性LLZTO电解质界面相的作用机理[94]。(b)PAN及不同LLZTO含量的PAN的1H NMR谱;(c)锂离子在复合电解质中粒子间传输的示意图[95]Fig. 6 ( a ) The function mechanism of PAN-modified SCN electrolyte interphase on the surface of LLZTO electrolyte[94]. Copyright 2021, Wiley ( b )1H NMR spectra of PAN and PAN with different amounts of LLZTO; ( c ) Schematic illustration showing the interparticle Li+ transport in the bulk of the composite electrolyte[95]. Copyright 2021, American Chemical Society |
图7 (a)非均质多层固体电解质示意图[96];(b)具有原始LATP和DPCE的固体全电池示意图[97];(c)NCM622‖非均质双层电解质膜‖Li电池原理图[98];(d)SPE膜的制备工艺示意图[99];(e)双层UFF/ PEO/PAN/LiTFSI SPE膜的制备图[100]Fig. 7 (a) Schematic diagram of the heterogeneous multilayered solid electrolyte[96]. Copyright 2019, Wiley (b) Illustrations of the solid full battery with pristine LATP and DPCE[97]. Copyright 2019, American Chemical Society. (c) Schematic diagram of the NCM622‖heterogeneous dual-layered electrolyte membrane‖Li battery[98]. Copyright 2021, Elsevier. (d) Schematic illustration of the preparation process for SPE membrane[99]. Copyright 2021, Elsevier. (e) The preparation diagram of the double-layer UFF/ PEO/PAN/LiTFSI SPE[100]. Copyright 2021, Wiley |
图8 固态电池微润湿设计示意图,SPE是薄而高强度的注入PEO/LiTFSI电解质的PAN膜(PLN)。(a)电池组件示意图;(b)液体电解质的位置和蒸气产生过程;(c)在PLN内部和PAN/PEO界面形成快速离子传输通道的混合溶剂;(d)PAN网络对TFSI-离子的吸附;(e)在阳极/电解液界面处产生的电解液蒸气分解产物LiPO2 [103]Fig. 8 Schematic illustrations of the micro-wetting design in a solid- state battery using thin and high-strength PAN network infused with PEO/LiTFSI electrolyte (PLN). (a) A schematic diagram of the battery assembly; (b) the position of the liquid electrolyte and the vapor generation process; (c) the mixed solvent forming fast-ion-transport channels at the internal PAN/PEO interface inside PLN; (d) the adsorption of TFSI- anions by the PAN network; (e) LiPO2F2, as the decomposition product of the electrolyte vapor, is generated at the external anode/electrolyte interface[103]. Copyright 2021, Royal Society of Chemistry |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
(杨琪, 邓南平, 程博闻, 康卫民. 化学进展, 2021, 33(12): 2270.).
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
/
| 〈 |
|
〉 |