

Development of Na-Based Seawater Batteries: “Key Components and Challenges”
Received date: 2022-09-02
Revised date: 2023-01-03
Online published: 2023-02-20
Supported by
National Natural Science Foundation of China(12175089)
National Natural Science Foundation of China(12205127)
Key Research and Development Program of Yunnan Province(202103AF140006)
Applied Basic Research Programs of Yunnan Provincial Science and Technology Department(202001AW070004)
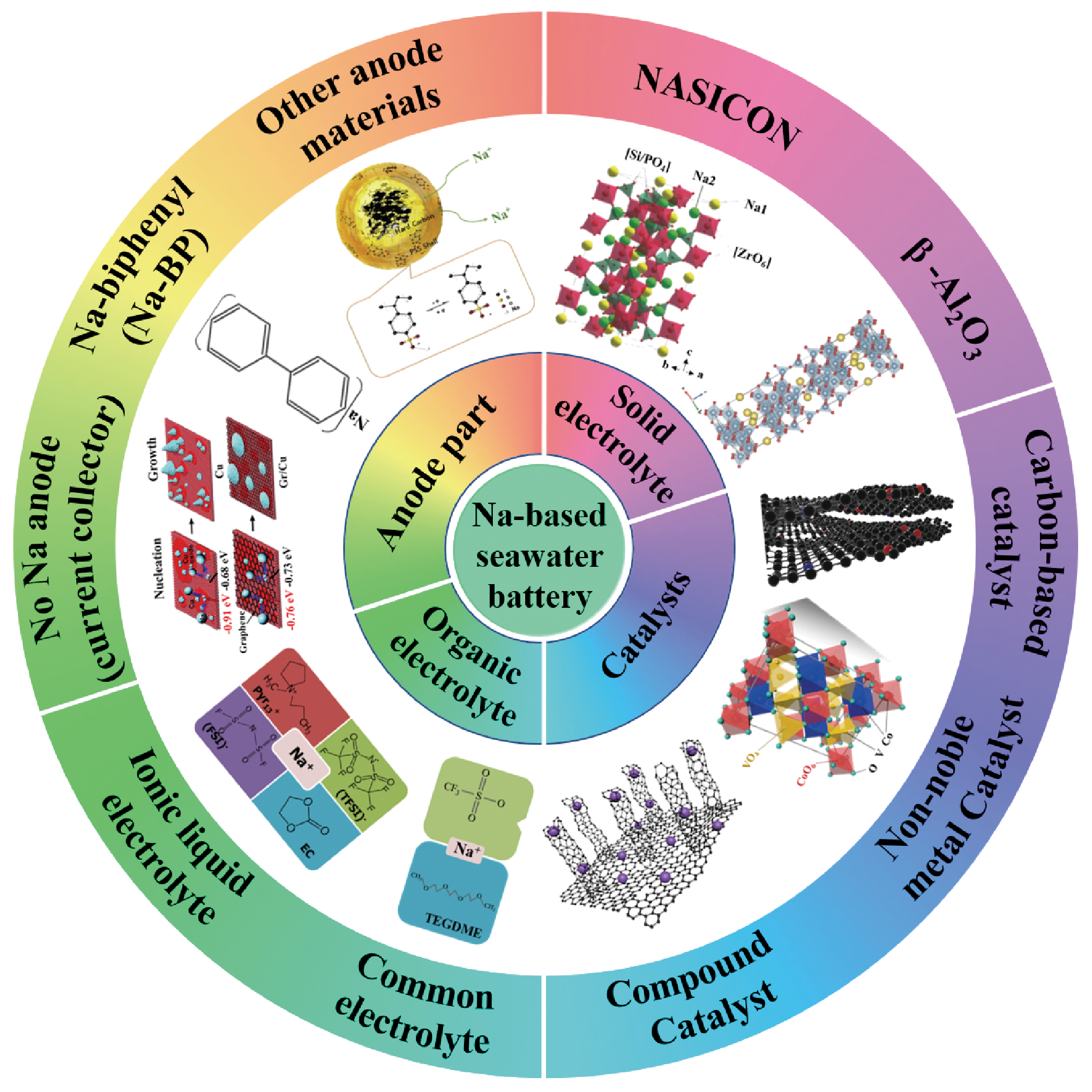
Na-based seawater batteries are expected to become a new-generation of energy storage device due to its advantages of environmental friendliness, high energy density, and abundant and easy availability of seawater. Its working principle is that the conversion between the chemical energy and the electrical energy is achieved through redox reaction when seawater is considered as the electrolyte. In this review, the electrochemical principle, and design and optimization strategy of battery structure of Na-based seawater batteries are summarized. The latest research progress of Na-based seawater batteries is reviewed. Finally, the challenges to overcome the performance improvement and commercialization of Na-based seawater batteries are discussed, and the future development directions of the batteries are forecasted. The review provides the theoretical guidance for the development of Na-based seawater batteries, and then promotes Na-based seawater batteries support for major national needs such as the deep-sea energy supply and extremely environmental-energy source.
Niu Wenhui , Zhang Da , Zhao Zhengang , Yang Bin , Liang Feng . Development of Na-Based Seawater Batteries: “Key Components and Challenges”[J]. Progress in Chemistry, 2023 , 35(3) : 407 -420 . DOI: 10.7536/PC220902
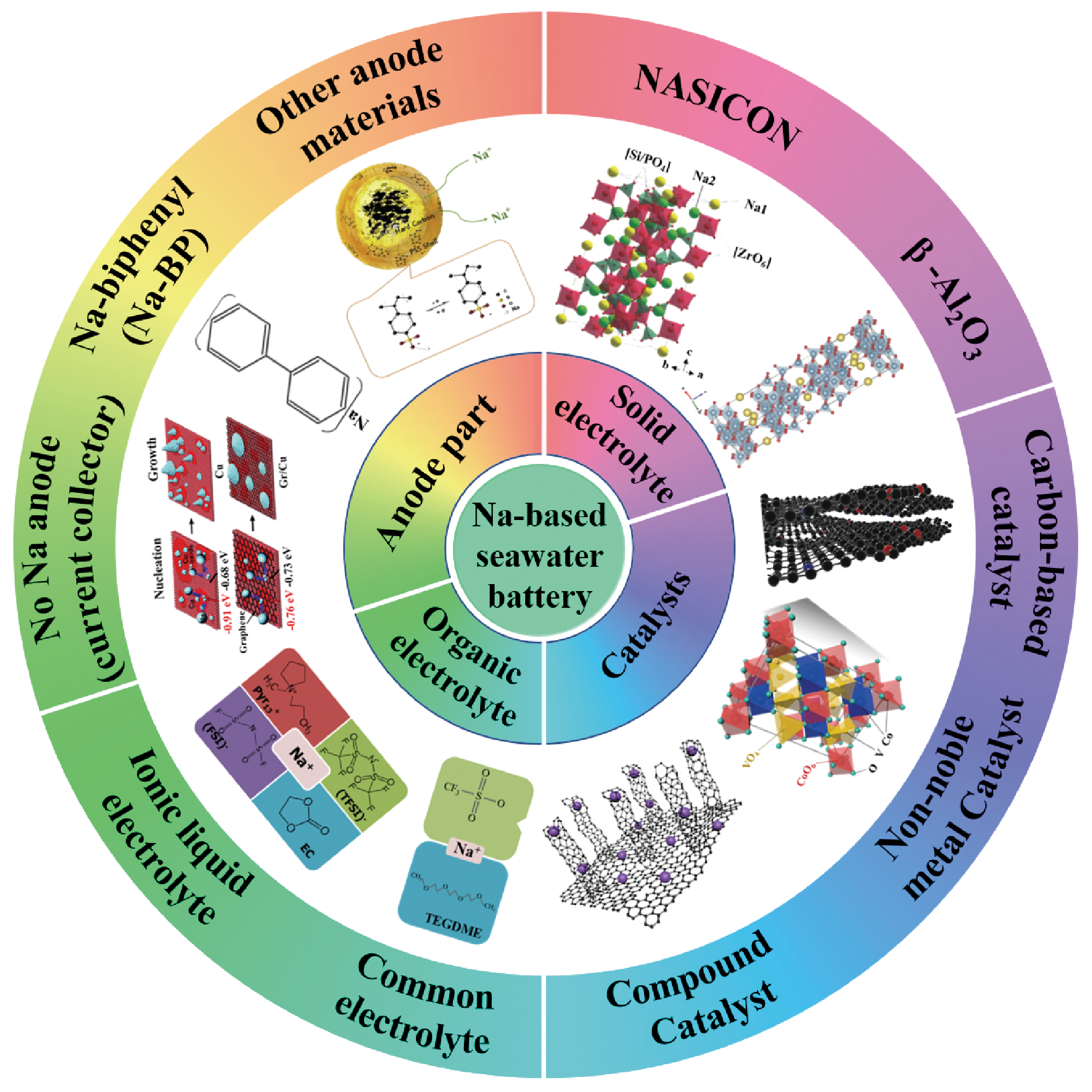
图4 (a)Cu集流体上钠枝晶生长的延时照片,黄色圆圈表示钠枝晶出现;(b)电池设计和钠枝晶生长示意图;(c)Cu和(d)Gr/Cu集流体的俯视扫描电子显微镜图像,插图表示集流体的示意图;(e)Gr/Cu集流体的高分辨率扫描隧道显微镜表面图像;(f)Gr/Cu集流体上钠枝晶生长的延时照片,黄色圆圈表示钠枝晶出现;(g)以Gr/Cu(红线)和Cu(黑线)为集流体的计时电位法图;(h)以Cu和Gr/Cu为集流体的钠基-海水电池充放电结构示意图[57]Fig. 4 (a) Time-lapse photographs of Na dendrite growth on the Cu current collector, the yellow circle shows the appearance of Na dendrite. (b) Schematic of the battery design and Na dendrite growth, top-view scanning electron microscope (SEM) images of (c) Cu and (d) Gr/Cu current collectors, the insets represent current collectors. (e) High resolution scanning tunneling microscope (STM) topography image of Gr/Cu current collector. (f) Time-lapse photographs of Na dendrite growth on the Gr/Cu current collector. the yellow circle shows the appearance of Na dendrite. (g) Chronopotentiometry plots of Gr/Cu (red line) and Cu (black line) current collectors. (h) Schematic diagram of charge-discharge of Na-base seawater battery with Cu and Gr/Cu current collectors[57] |
图5 (a)Na-BP-DEGDME阳极电池充放电过程示意图;(b)Na-BP-DEGDME阳极钠基-海水电池循环性能图[59];(c)在0.01 mA·cm-2下,Na-BP-TEGDME阳极电池的循环性能和能量效率图;(d)在0.01 mA·cm-2下,Na-BP-TEGDME阳极电池的恒电流循环曲线;(e)使用不同催化剂和Na-BP-TEGDME阳极电池的能量密度图[35]Fig. 5 (a) Schematic diagram of charging and discharging process of Na-BP-DEGDME anode battery. (b) Cycle performance of Na-based seawater battery with Na-BP-DEGDME anode[59]. (c) Cycling performance and energy efficiency of battery with Na-BP-TEGDME anode at 0.01 mA·cm-2. (d) Galvanostatic cycling of battery with Na-BP-TEGDME anode at 0.01 mA·cm-2. (e) Power density of batteries with different catalysts and Na-BP-TEGDME anode[35] |
表1 使用有机电解液对应的钠基-海水电池电化学性能的对比Table 1 Comparison of electrochemical performance of Na-based seawater batteries with organic electrolyte |
| Anodic electrolyte | Anode | Applied current | Discharge capacity (mAh·g-1) | Cyclic performance | ref |
|---|---|---|---|---|---|
| 1 M NaClO4 in EC/PC | HC | 0.05 mA·cm-2 | 115 | 30 | 63 |
| 1 M NaCF3SO3 in TEGDME | HC | 0.05 mA·cm-2 | 126 | 100 | 63 |
| ILE-EC | HC | 0.3 mA·cm-2 | 290 | 600 | 46 |
| 1 M NaClO4 in EC/DMC (1∶1) with 1 vol% FEC | PC | 1.0 A·g-1 | 973 | 80 | 67 |
| 1 M NaClO4 in EC/DMC (1∶1) | Sn-C | 0.05 mA·cm-2 | 300 | 30 | 68 |
图6 海水电池使用两种电解液(1 M NaCF3SO3溶于TEGDME,1 M NaClO4溶于EC/PC)的(a)充/放电曲线和(b)循环性能[63];(c)使用电解液ILE-EC的钠基-海水电池容量保持率[46];(d)使用电解液ILE-EC和1 M NaCF3SO3溶于TEGDME的钠基-海水电池循环性能[56]Fig. 6 (a) Charge-discharge curves and (b) Cycling performance of the seawater battery with 1 M NaCF3SO3 in TEGDME and 1 M NaClO4 in EC/PC electrolyte[63]. (c) Capacity retention of Na-based seawater battery with ILE-EC electrolyte[46]. (d) Cycling performance of Na-based seawater battery with ILE-EC and 1 M NaCF3SO3 in TEGDME electrolyte[56] |
图7 (a)β-Al2O3和β″-Al2O3固体电解质的晶体结构[75];(b)钠基-海水电池循环前后β″-Al2O3固体电解质的XRD图[43];(c)NASICON(Na3Zr2Si2PO12)固体电解质的晶体结构和离子迁移路径[83];(d)NASICON固体电解质在海水浸泡前后的XRD图和SEM图[13];(e)NASICON固体电解质在不同pH值溶液中浸泡不同时间的离子电导率;(f)NASICON固体电解质在不同pH值酸性溶液中的腐蚀机理[86]Fig. 7 (a) Crystal structures of the β-Al2O3,and β″-Al2O3 solid electrolytes[75]. (b) X ray diffraction (XRD) patterns of β″-Al2O3 solid electrolytes before and after cycling of Na-based seawater battery[43]. (c) Crystal structures and ion transport paths of NASICON (Na3Zr2Si2PO12) solid electrolytes[83]. (d) XRD patterns and SEM images of NASICON solid electrolytes before and after immersion in seawater[13]. (e) Ionic conductivity of NASICON solid electrolytes with immersion different time in solutions with different pH values. (f) Corrosion mechanism of NASICON solid electrolyte in acidic solutions with different pH values[86] |
表2 不同催化剂对应的钠基-海水电池电化学性能对比Table 2 Comparison of electrochemical performance of Na-based seawater batteries with different catalyst |
| Catalysts | Anode | Electrolyte | Applied current | Voltage gap (V) | Discharge capacity (mAh·g-1) | Cyclic performance | ref |
|---|---|---|---|---|---|---|---|
| CMO | HC | 1 M NaCF3SO3 in TEGDME | 0.01 mA·cm-2 | ~0.53 | ~190 | 100 | 95 |
| 3D macroporous carbon sponge | Na | 1 M NaCF3SO3 in TEGDME | 0.025 mA·cm-2 | 0.46 | - | 100 | 102 |
| N,S-doped carbon nanospheres | Na | 1 M NaCF3SO3 in TEGDME | 5 mA·g-1 | 0.56 | - | 100 | 103 |
| Pine pollen carbon (PPC) | PPC | 1 M NaCF3SO3 in TEGDME | 50 mA·g-1 | ~195 | 100 | 93 | |
| Activated carbon cloth | Na | 1 M NaCF3SO3 in TEGDME | 0.13 mA·cm-2 | ~0.6 | - | 80 | 43 |
| Co3V2O8 | Na | 1 M NaCF3SO3 in TEGDME | 0.1 mA·cm-2 | ~0.9 | - | 20 | 96 |
| S-rGO-CNT-Co | Na | 1 M NaCF3SO3 in TEGDME | 0.01 mA·cm-2 | ~0.42 | - | 50 | 100 |
| Porous carbon | HC | 1 M NaCF3SO3 in TEGDME | 0.01 mA·cm-2 | 0.47 | ~191 | 100 | 91 |
| PPy+Co3O4@CF | Na | 1 M NaCF3SO3 in TEGDME | 20 mA·g-1 | ~0.95 | - | 150 | 101 |
| Pyridinic-nitrogen-containing carbon | Na/CC | 1 M NaCF3SO3 in DME | 0.25 mA·cm-2 | 0.84 | - | 20 | 104 |
| Co-N/C | Na | 1 M NaCF3SO3 in TEGDME | 0.1 mA | 0.54 | - | 100 | 105 |
图8 (a)在0.01 mA·cm-2下,使用PC催化剂的钠基-海水半电池充放电曲线。插图为PC的SEM图[91];(b)在50 mA· 下,以PPC为催化剂的钠基-海水电池循环性能[93];(c)在0.01 mA·cm-2下,以CMO为催化剂的钠基-海水半电池充放电曲线,插图为PPC的SEM图[95];(d)在0.1 mA·cm-2下,使用Co3V2O8催化剂的钠基-海水电池循环性能[96];(e)在0.01 mA·cm-2下,使用S-rGO-CNT-Co催化剂的钠基-海水电池充放电曲线,插图为S-rGO-CNT-Co的SEM图[100];(f)在20 mA·g-1下,使用PPy+Co3O4@CF催化剂的钠基-海水电池循环性能[101]Fig. 8 (a) Charge-discharge curves of Na-based seawater half-battery with PC catalyst at 0.01 mA·cm-2. Inset is SEM image of PC[91]. (b) Cycling performance of Na-based seawater battery with PPC catalyst at 50 mA· [93]. (c) Charge-discharge curves of Na-based seawater half-battery with CMO catalyst at 0.01 mA·cm-2. Inset is SEM image of CMO[95]. (d) Cycling performance of Na-based seawater battery with Co3V2O8 catalyst at 0.1 mA·cm-2[96]. (e) Charge-discharge curves of Na-based seawater battery with S-rGO-CNT-Co catalyst at 0.01 mA·cm-2. Inset is SEM image of S-rGO-CNT-Co[100]. (f) Cycling performance of Na-based seawater battery with PPy+Co3O4@CF catalyst at 20 mA·g-1[101] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
/
| 〈 |
|
〉 |