

Photovoltaic Cells Based on Carbon Nanotubes
†These authors contributed equally to this work.
Received date: 2022-11-08
Revised date: 2023-01-03
Online published: 2023-02-16
Supported by
National Natural Science Foundation of China for Excellent Young Scholars(61622404)
National Natural Science Foundation of China(62074098)
Chang Jiang (Cheung Kong) Scholars Program of Ministry of Education of China(Q2017081)
Project from Zhejiang Fulai New Materials Co., Ltd.
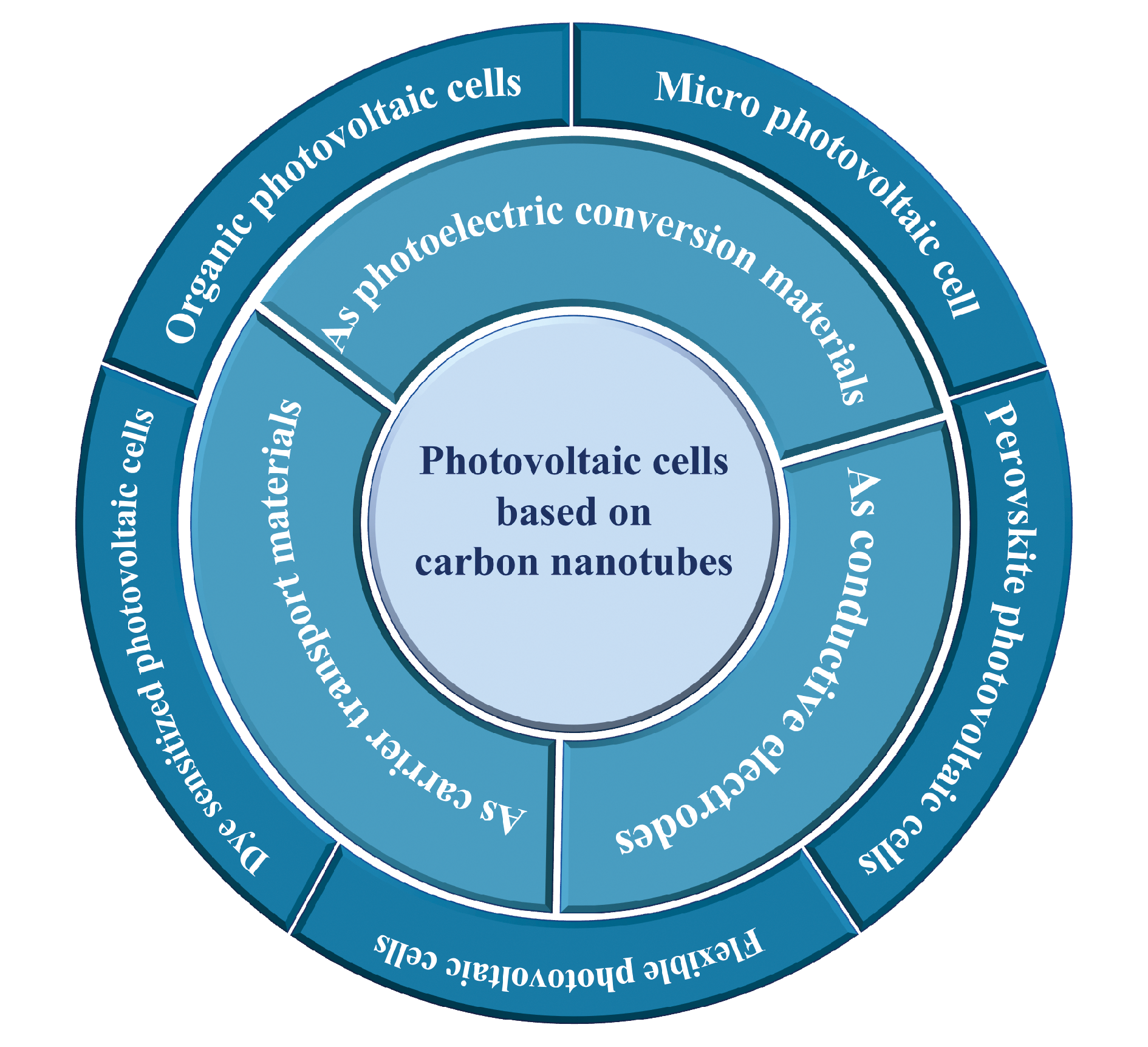
Carbon nanotubes (CNTs) are ideal materials for building photovoltaic cells due to their unique one-dimensional structure and excellent photoelectric properties. In this paper, we review recent structural design, fabrication method and device performance of CNT-based photovoltaic cells and different functional roles of CNTs in these devices. Firstly, the structure and photoelectric properties of CNTs are introduced. Then, we emphatically discuss the operation principles, the fabrication methods and the advantages and shortage of the photovoltaic cells with CNTs used as the photoelectric conversion materials, conducting electrodes and carrier transport layers in the devices. The applications of carbon nanotubes in Micro photovoltaic cell,carbon nanotube/silicon heterojunction photovoltaic cells, dye sensitized photovoltaic cells, perovskite photovoltaic cells, organic photovoltaic cells and flexible photovoltaic cells are introduced. Finally, the advantages and challenges of CNT-based photovoltaic cells are summarized. This paper will provide new idea and reference for the design and fabrication of novel carbon-based photovoltaic cells.
Wang Long , Zhou Qingping , Wu Zhaofeng , Zhang Yanming , Ye Xiaowo , Chen Changxin . Photovoltaic Cells Based on Carbon Nanotubes[J]. Progress in Chemistry, 2023 , 35(3) : 421 -432 . DOI: 10.7536/PC221106
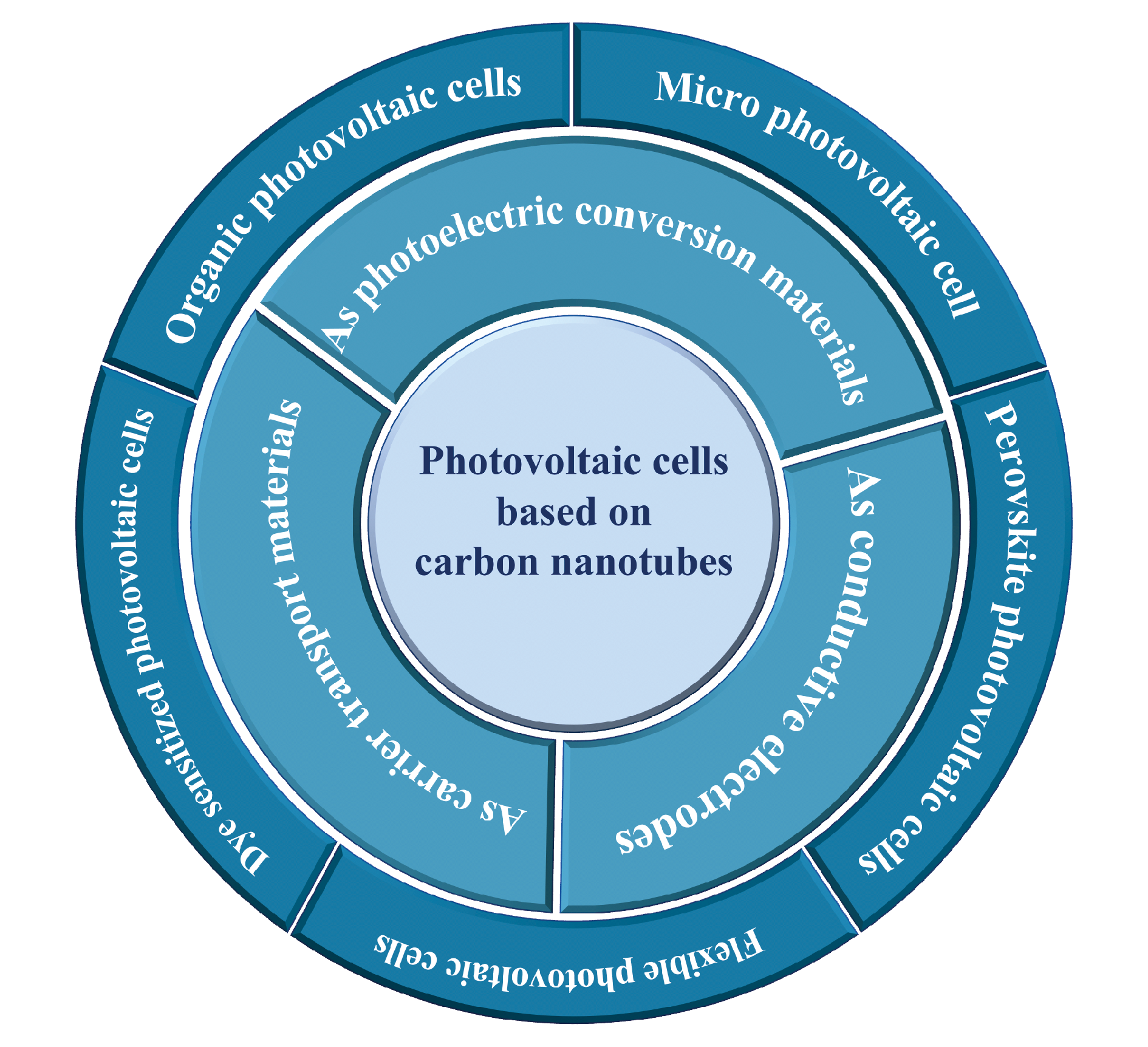
图4 (a)典型的碳纳米管/硅异质结太阳能电池结构[45];(b)气体掺杂结构示意图[47];(c)氧化钨掺杂的碳纳米管/硅异质结光伏电池结构[48];(d)碳纳米管/硅异质结光伏电池载流子传输途径示意图[48]Fig. 4 (a) Typical CNT/silicon heterojunction solar cell structure[45]. (b) Schematic Diagram of gas doping Structure[47]. (c) Tungsten oxide doped CNT/silicon heterojunction photovoltaic cell structure[48]. (d) Schematic diagram of carrier transport paths of CNT/silicon heterojunction photovoltaic cells[48] |
图6 (a)碳纳米管透明导电电极的染料敏化光伏电池结构示意图;(b)电极层表面形貌图;(c)不同电极下DSSC的J-V曲线;(d)不同对电极下DSSC的IPCE曲线[63]Fig. 6 (a) Structure diagram of DSSC cell with CNT transparent conductive electrode. (b) Surface topography of electrode layer. (c) J-V curve of DSSC with different electrodes. (d) IPCE curve of DSSC under different pair electrodes[63] |
图7 (a)使用Mo2C-CNTs@PEDOT:PSS作为 HTL的光伏电池结构;(b)使用Mo2C-CNTs@PEDOT:PSS作为 HTL的钙钛矿光伏电池的能级图;(c)碳纳米管质量分数分别为1%(紫),1.5%(绿),2.0%(蓝)PSC的J-V曲线;(d)不同组分的HTL下(HTL中未加入Mo2C-CNTs(黑)、只加入Mo2C(红)、只加入CNTs(蓝)、加入Mo2C-CNTs(绿)的),PSC的外部量子效率(EQE)谱[75]Fig. 7 (a) PV cell structure using Mo2C-CNTs@PEDOT:PSS as HTL. (b) Energy level diagram of perovskite photovoltaic cells using Mo2C-CNTs@PEDOT:PSS as HTL. (c) J-V curve of PSC with 1% (purple), 1.5% (green) and 2.0% (blue) carbon nanotubes. (d) External quantum efficiency (EQE) spectrum of PSC under HTL with different components (no Mo2C-CNTs (black), only Mo2C (red), only CNTs (blue), and Mo2C-CNTs (green) added in HTL) [75] |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
in het Panhuis M. J. Mater. Chem., 2006, 16(36): 3598.
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
/
| 〈 |
|
〉 |