

MIL-101(Fe) and Its Composites for Catalytic Removal of Pollutants: Synthesis Strategies, Performances and Mechanisms
Received date: 2022-08-26
Revised date: 2023-01-08
Online published: 2023-02-15
Supported by
National Natural Science Foundation of China(22176012)
Beijing Natural Science Foundation(8202016)
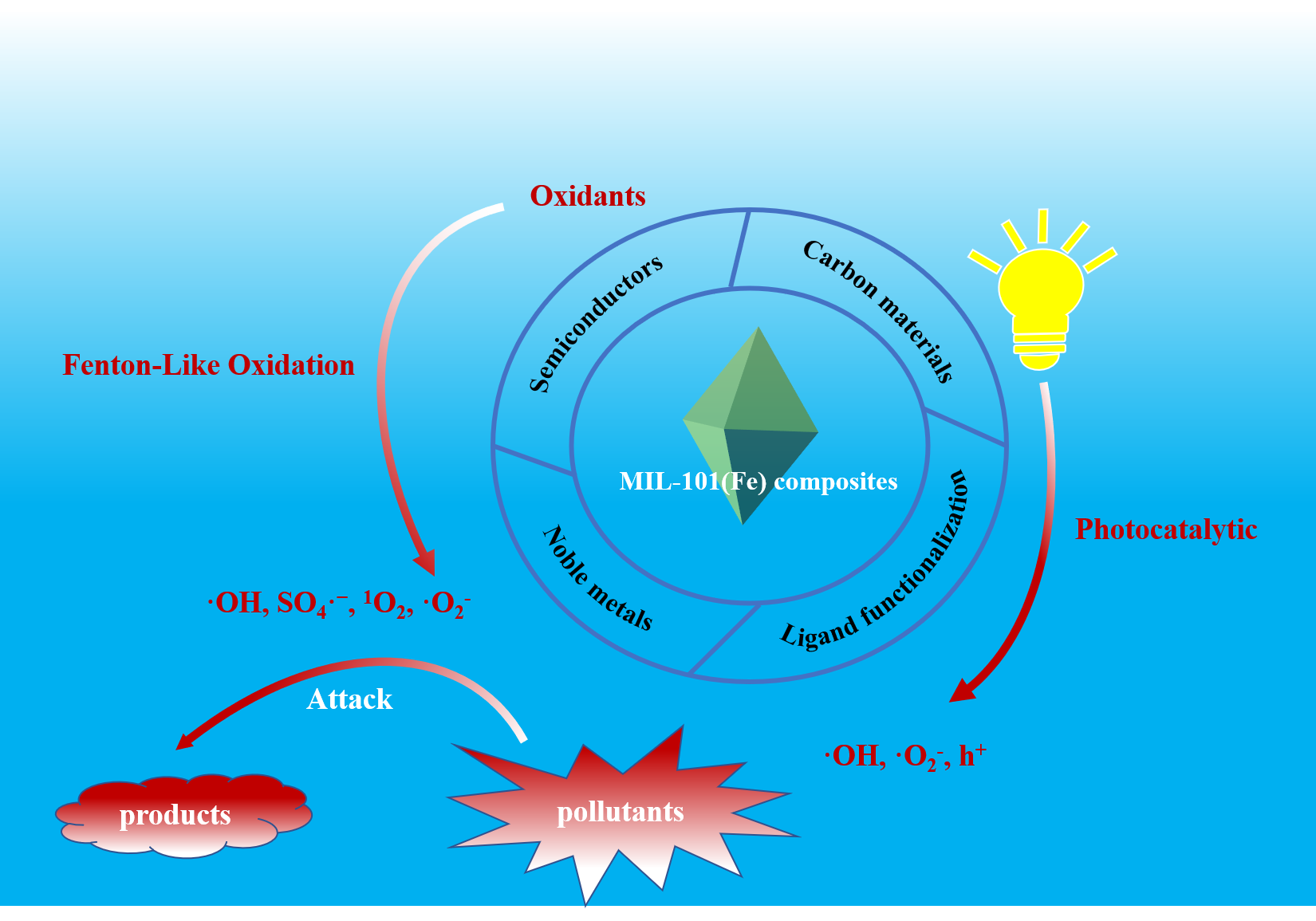
MIL-101(Fe) is a typical Fe-based metal-organic framework (Fe-MOF), which demonstrates the advantages of flexible structure, large specific surface area, large porosity, and adjustable pore size. In recent years, MIL-101(Fe) and its composites have been extensively studied in the field of water pollution remediation, especially in the hexavalent chromium (Cr(Ⅵ)) reduction and advanced oxidation processes for removing organic pollutants in water. The water stability, light absorption activity and the carrier separation efficiency can be significantly improved by functional modification with specific functional materials. In this review, the preparation strategies of MIL-101(Fe) and its composites, as well as their application as heterogeneous catalysts for photocatalysis, H2O2 activation, and persulfate activation were introduced. The future development of MIL-101(Fe) and its composites as catalysts for water purification is prospected.
Lan Mingyan , Zhang Xiuwu , Chu Hongyu , Wang Chongchen . MIL-101(Fe) and Its Composites for Catalytic Removal of Pollutants: Synthesis Strategies, Performances and Mechanisms[J]. Progress in Chemistry, 2023 , 35(3) : 458 -474 . DOI: 10.7536/PC220822
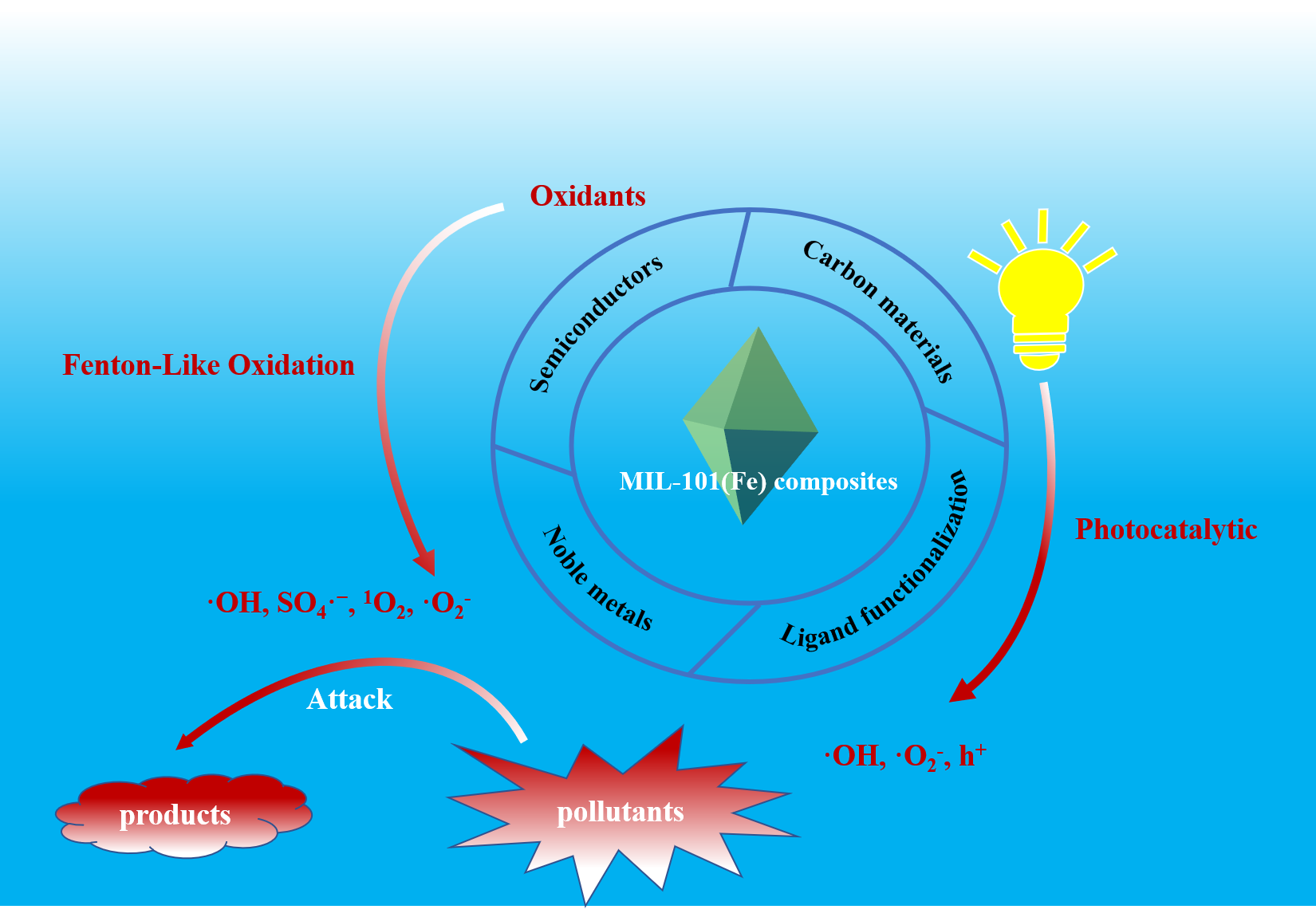
图2 (a) 溶剂热法[18];(b) 微波辅助法[21];(c) 电化学法[23]和(d) 室温搅拌法[24]合成的MIL-101(Fe)形貌图;(e) 原位合成法合成的MIL-101(Fe)/g-C3N4复合材料[27];(f) 一步合成法合成的MIL-101(Fe)/CuS复合材料[30]和(g) 室温浸渍法合成的Ag/AgCl/MIL-101(Fe)复合材料的形貌图[32]Fig. 2 The morphologies of MIL-101(Fe) synthesized via (a) solvothermal method[18]; (b) microwave-assisted method[21]; (c) electrochemical method[23] and (d) room temperature method; the morphologies of (e) MIL-101(Fe)/g-C3N4 composite[27]; (f) MIL-101(Fe)/CuS composite[30] and (g) Ag/AgCl/MIL-101(Fe) composite[32] synthesized via in-situ synthesis, one-step synthesis and room temperature impregnation, respectively |
表1 MIL-101(Fe)及其复合物用于光催化还原Cr(Ⅵ)Table 1 MIL-101(Fe) and its composites for photocatalytic Cr(Ⅵ) reduction |
| Catalyst/Dosage (g·L-1) | Volume (mL)/ Concentration (mg·L-1)/pH | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|
| NH2-MIL-101(Fe)/0.5 | 40/80/2 | visible light | 60 | 100 | 44 |
| 150-g-C3N4/NH2-MIL-101(Fe)/0.5 | 40/10/2 | 300 W Xe lamp (λ≥ 400 nm) | 60 | 100 | 45 |
| MIL-101(Fe)/g-C3N4/0.5 | 40/20/5 | 150 W halogen cold light source (λ≥ 420 nm) | 60 | 92.6 | 27 |
| 1%Ag/AgCl/MIL-101(Fe/)1 | 50/10/6 | 300 W Xe lamp (λ≥ 420 nm) | 75 | 100 | 32 |
| Cellulose/NH2-MIL-101(Fe) hybrid foams/1 | 40/20/5 | light intensity: 100 mW/cm2(λ≥ 420 nm) | 180 | 100 | 46 |
| Sand-Cl@NH2-MIL-101(Fe)-50%/0.5 | 100/10/ | 1000 W halogen lamp | 20 | 97.3 | 47 |
| g-C3N4 (150 mg)/ NH2-MIL-101(Fe)/1 | 30/20/2 | solar light (60,000 lux) | 90 | 91 | 48 |
| MIL-101(Fe)-NH2@Al2O3/0.3 | 50/5/3.4 | 300 W Xe lamp | 180 | 100 | 42 |
| TmErNd@Nd(x)@NFM/0.5 | 40/20/2 | 300 W Xe lamp | 50 | 91 | 49 |
图3 (a) 不同材料光催化还原Cr(Ⅵ)的性能图[44];(b) 在草酸存在下MA光催化还原Cr(Ⅵ)的机理图[42];(c) g-C3N4/NH2-MIL-101(Fe)光催化还原Cr(Ⅵ)的机理图[45];(d) Ag/AgCl/MIL-101(Fe)光催化还原Cr(Ⅵ)的机理图[32]Fig. 3 (a) Performances of photocatalytic Cr(Ⅵ) reduction over different materials[44]; schematic illustration of photocatalytic Cr(Ⅵ) reduction mechanism of (b) MA[42]; (c) g-C3N4/NH2-MIL-101(Fe)[45]; (d) Ag/AgCl/MIL-101(Fe)[32] |
图4 (a) 可见光照射下MIL-101(Fe)中光生电子-空穴对的分离和转移示意图[59];(b) m-MIL-101-1.0的制备过程示意图[64];(c) m-MIL-101-1.0中e-和h+的转移过程和光催化机理图[64];(d) MIL-101(Fe)/WO3光催化降解TCH机理图[74];(e) TiO2/MIL-101(Fe)的透射电子显微镜图[63];(f) CFs/TiO2/MIL-101(Fe)的吸附及光催化示意图[63]Fig. 4 (a) Schematic diagram of the separation and transfer of photo-generated electron-hole pairs in MIL-101(Fe) under visible light irradiation[59]; (b) schematic diagram of the preparation process of m-MIL-101-1.0[64]; (c) schematic diagram of the transfer process and photocatalytic mechanism of e- and h+ in m-MIL-101-1.0[64]; (d) proposed charge separation process and catalytic mechanism for TCH photodegradation over MIL-101(Fe)/WO3 hybrid system[74]; (e) TEM image of TiO2/MIL-101(Fe)[63]; (f) schematic diagram of adsorption and photocatalytic mechanism of CFs/TiO2/MIL-101(Fe)[63] |
表2 MIL-101(Fe)及其复合物用于光催化降解有机污染物Table 2 MIL-101(Fe) and its composites for photocatalytic organic pollutants degradation |
| Catalyst/dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|
| MIL-101(Fe)/0.5 | tetracycline/100/50/- | 300 W Xe lamp (λ≥ 420 nm) | 180 | 96.6 | 59 |
| V2O5/NH2-MIL-101(Fe)-10/0.5 | tetracycline/100/-/- | ultraviolet-visible light from a 300 W xenon lamp | 120 | 88.3 | 60 |
| NH2-MIL-101(Fe)/Cu2O-2/1 | rhodamine B/100/4.8/- | 300 W Xe lamp (λ≥ 420 nm) | 90 | 92 | 61 |
| Electrospun graphene oxide/MIL-101(Fe)/poly (acrylonitrile-co-maleic acid) nanofiber/2 | rhodamine B/20/-/- | ultraviolet lamp (16 W) | 20 | 93.7 | 62 |
| carbon fibers/TiO2/MIL-101(Fe)/2 | 17β-estradiol/100/3/-;tetracycline/100/20/- | visible light | 60 | 87.4 (17β-estradiol)/94.2 (tetracycline) | 63 |
| m-MIL-101-1.0/0.5 | tetracycline/20/20/- | 300 W Xe lamp (λ≥ 420 nm) | 60 | 85.41 | 64 |
| Magnetic MIL-101(Fe)/TiO2/1 | tetracycline/50/20/7 | solar light | 10 | 92.76 | 65 |
| 5-Bi2MoO6/MIL-101(Fe)/0.3 | rhodamine B/100/15/6.5 | blue light LED | 83.2 | 90 | 66 |
| MIL-101(Fe)/gC3N4/0.5 | bisphenol A/40/10/6.8 | 150 W halogen cold light source (λ≥ 420 nm) | 240 | 94.8 | 27 |
| 1%Ag/AgCl/MIL-101(Fe/)1 | phenol/50/10/6 | 300 W Xe lamp (λ≥ 420 nm) | 30 | 70 | 32 |
| g-C3N4/NH2-MIL-101(Fe)/1 | 2,6-dichlorophen/30/10/- 2,4,5-trichlorophenol/30/10/- | 300 W Xe-lamp | 180 | 98.7 (2,6- dichlorophen)/ 97.3 (2,4,5- trichlorophenol) | 67 |
| Cu2O/Fe3O4/MIL-101(Fe)/0.5 | ciprofloxacin//20/7 | 500 W Xe lamp | 105 | 99.2 | 43 |
| NCQDs/MIL-101(Fe)/0.5 | tetracycline/100/10/- | 500 W Xe lamp (λ≥ 420 nm) | 180 | 100 | 68 |
| g-C3N4@NiO/Ni-3@MIL-101/0.01 | ibuprofen/30/30/- | 500W Xenon (λ>400 nm) | 120 | 95.6 | 69 |
| Tm@Yb@Y/NMF/0.03 | tetracycline/levofloxacin/ rhodamine B/60/20/- | 500 W Xe lamp | 50 | 47 (tetracycline)/ 70 (levofloxacin)/ 77 (rhodamine B) | 70 |
| NH2-MIL-101(Fe)/Ti3C2Tx/1 | phenol/chlorophenol/100/23.5/- | 300 W Xe lamp (λ≥ 420 nm) | 60 | 99.36 (phenol)/ 99.83 (chlorophenol) | 71 |
表3 MIL-101(Fe)及其复合物用于活化H2O2降解有机污染物Table 3 MIL-101(Fe) and its composites for organic pollutants degradation via activation of H2O2 |
| Catalyst/dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | H2O2 dosage | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|---|
| MIL-101(Fe)/0.1 | phenol/150/50/4 | 15 mM | in dark | 30 | 62 | 83 |
| Fe3O4/MIL-101(Fe)/0.5 | rhodamine B/100/10/7 | 20 mM | in dark | 30 | 100 | 85 |
| NH2-MIL-101(Fe)/0.1 | rhodamine B/50/0.025 mM/7.22 | 0.5 mL | in dark | 4 | 100 | 86 |
| GA/MIL-101(Fe)/0.1 | phenol/50/0.1 mM/5 | 6 mM | in dark | 40 | 99 | 84 |
| MIL-101(Fe,Cu)/0.1 | ciprofloxacin/100/20/7 | 3 mM | in dark | 30 | 100 | 87 |
| NH2-MIL-101(Fe) -EPU/0.5 | tetrabromobisphenol A/20/1.84 mM/3 | 165 mM | light-emitting diodes (λ≥ 400 nm) | 120 | 120 | 88 |
| MIL-101(Fe,Co)/0.2 | ciprofloxacin/100/20/5 | 5 mM | in dark | 30 | 97.8 | 89 |
| NH2-MIL-101(Fe)/0.2 | bisphenol A/50/50/6 | 10 mM | in dark | 30 | 100 | 24 |
| MIL-101 (Fe)/PANI/Pd/0.05 | methylene Blue/-/25/7 | 1 M | - | 34 | 92 | 90 |
| MoS2@NH2-MIL-101(Fe)/0.2 | rhodamine B/50/50/- bisphenol A/50/20/- | 1.76 mM | 300 W Xe lamp | 10 | 97.4 (RhB) 99.9 (BPA) | 91 |
| Fe/Ce-MIL-101/0.3 | norfloxacin/-/10/7 | 20 mM | in dark | 60 | 94.8 | 92 |
| TiO2@17%NH2-MIL-101(Fe)/1 | methylene Blue/100/50/- | - | 300 W Xe lamp (λ≥ 420 nm) | 30 | 96 | 93 |
| CNT@MIL-101(Fe)/0.5 | ciprofloxacin/100/3.02 μM/3 | 165 mM | white light LEDs, 360-830 nm | 45 | 90 | 94 |
| GO@MIL-101(Fe)/0.5 | tris(2-chloroethyl) phosphate/-/3.51μM/3 | 165 mM | multiple wavelength LEDs | 30 | 95 | 95 |
| AFG@30MIL-101(Fe)/0.4 | diazinon/50/30/9 atrazine/50/30/2 | 1.5 mL | high-pressure mercury- vapor lamp (400 W and λ = 546.8 nm) | 120 | 100 (diazinon) 81 (atrazine) | 96 |
| MIL/Co/(3%)GO/0.2 | direct Red 23/-/100/3 reactive Red 198/-/100/3 | 50 μL | 100 W LED projector | 70 | 99.93 (Direct Red 23) 99.65 (Reactive Red 198) | 97 |
| MIL-101(Fe)@Zn/Co-ZIFs/0.2 | rhodamine B/50/100/5 | 90 mM | 350 W Xe lamp (λ≥ 420 nm) | 180 | 98 | 98 |
| MIL-101(Fe)/Bi2WO6/Fe(Ⅲ)/ 0.5 | methylene Blue/100/20/- | 500 μL | 200 W incandescent lamp | 75 | 86.7 | 99 |
| MIL-101(Fe)-NH2@Al2O3/0.3 | norfloxacin/50/10/- | 15 μL | 350 W Xe lamp | 97.3 | 100 | 80 |
图5 (a) GA/MIL-101(Fe)的制备策略示意图[84];(b) Fe-BDC-NH2/H2O2系统催化降解BPA的机理图[24];(c) CUMSs/MIL-101(Fe, Cu)/H2O2系统催化降解CIP的机理图[87];(d) MIL-101(Fe)/H2O2/vis系统催化降解TC—HCl的机理图[103]Fig. 5 (a) Schematic diagram showing the design strategy of GA/MIL-101(Fe)[84]; (b) schematic diagram of the proposed mechanisms involved for BPA degradation in Fe-BDC-NH2/H2O2 system[84]; (c) schematic diagrams of the proposed mechanism involved in CIP degradation by CUMSs/MIL-101(Fe, Cu)/H2 [87]; (d) illustration of the proposed reaction mechanism for TC-HCl removal in MIL-101(Fe)/H2O2/visible light system[103] |
表4 MIL-101(Fe)及其复合物用于活化过硫酸盐降解有机污染物Table 4 MIL-101(Fe) and its composites for organic pollutants degradation via activation of persulfate |
| Catalyst/Dosage (g·L-1) | Polluant/Volume (mL)/ Concentration (mg·L-1)/pH | PS dosage | Light source | Reaction time (min) | Degradation efficiency (%) | ref |
|---|---|---|---|---|---|---|
| MIL-101(Fe)/0.625 | acid orange 7/25/80/6.16 | 15 mM | in dark | 120 | 95 | 108 |
| Fe3O4@MIL-101/1 | acid orange 7/10/25/3.58 | 25 mM | in dark | 60 | 98.1 | 109 |
| Quinone-modified NH2-MIL-101(Fe)/0.2 | bisphenol A/25/60/5.76 | 10 mM | in dark | 120 | 97.7 | 110 |
| 6 wt% Co-MIL-101(Fe)/0.2 6 wt% Cu-MIL-101(Fe)/0.2 | acid orange 7/100/0.1 mM/- | 8 mM | in dark | 180 | 92 (6 wt% Co-MIL-101(Fe)) 98 (6 wt% Co-MIL-101(Fe)) | 17 |
| g-C3N4/MIL-101(Fe)/0.5 | bisphenol A/-/10/- | 1 mM | 350 W Xe lamp (λ≥ 400 nm) | 60 | 98 | 111 |
| MIL-101(Fe) via vacuum thermal treatment/0.1 | X-3B/100/100/- | 15 mM | in dark | 180 | 95.7 | 112 |
| MIL-101(Fe)/0.5 | tris(2-chloroethyl) phosphate/20/3.51 μM/- | 500 mg·L-1 | light-emitting diodes (LEDs) with emission peaks | 180 | > 90 | 113 |
| MIL-101(Fe)/TiO2/1 | tetracycline/-/80/7 | 1 g·L-1 | 500 W Xe lamp | 30 | 93.02 | 114 |
| MIL-101(Fe)-NH2/1 | amaranth/200/50/7 | 200 mg·L-1 | 150 W visible light | 30 | 100 | 115 |
| NH2-MIL-101(Fe)/0.02 | bisphenol F/200/20/5 | 1 mM | in dark | 120 | 100 | 116 |
| MIL-101(Fe)/1 | methylene Blue/20/10/7 | 500 mg·L-1 | in dark | 25 | > 90 | 117 |
| N,S:CQD/MIL-101(Fe)/0.4 | bisphenol A/100/20/- | 3 mM | 350 W Xe lamp (λ≥ 400 nm) | 60 | 100 | 118 |
| CuS-modified MIL-101(Fe)/0.1 | E. coli/100/ 107.5 cfu· mL-1/6.5 | 50 μM | white LED lamps (11,000 Lux, 400~700 nm) | 40 | 100 | 30 |
| TiO2@MIL-101(Fe)/1.052 | nitrobenzene/28.5/800 μM/- | 1.6 mM | Xe lamp (λ≥ 420 nm) | 240 | 66.53 | 31 |
| RGO/MIL-101(Fe)/0.5 | trichlorophenol/-/20/3 | 20 mM | in dark | 180 | 92 | 119 |
| MIL-101(Fe)/0.1 | orange G/50/15/3 | 0.05 mM | in dark | 40 | 74 | 120 |
| MIL-101(Fe)/g-C3N4/0.08 | tetracycline hydrochloride/ 50/-/3.5 | 0.85 mM | 30-W LED lamp (λ=410~760 nm) | 40 | 99 | 121 |
| NH2-MIL-101(Fe)-ferrocene/0.2 | bisphenol A/25/60/5.76 | 10 mM | in dark | 40 | 100 | 122 |
| NH2-MIL-101(FeCo)-2/0.005 | orange G/99/0.2 nM/7 | 2 mM | in dark | 45 | 100 | 123 |
| M/Z2/0.01 | 2-chlorophenol/100/100/9 | 300 mg·L-1 | in dark | 10 | 90.3 | 124 |
图6 (a) MIL-101(Fe)活化PS催化降解AO7的机理图[108];(b) 分别使用HBC、HAC、OA和CA(Fe,深橙色;C,黑色;O,红色;H,白色)调控,合成缺陷MIL-101(Fe)催化剂的制备策略[128];(c) RGO/MIL-101(Fe)活化PS的反应机理图[119];(d) N, S: CQDs /MIL-101(Fe)/PS/vis体系中的光催化降解机理图[118]Fig. 6 (a) The possible elimination mechanism of AO7 by MIL-101(Fe)[108]; (b) scheme of the strategy for the syntheses of defective MIL-101(Fe) by modulating synthesis using HBC, HAC, OA, and CA, respectively (Fe, dark orange; C, black; O, red; H, white)[128]; (c) schematic diagram of the reaction mechanism of the PS activation by RGO/MIL101(Fe)[119]; (d) possible photocatalytic degradation mechanism in the N, S: CQD/MIL-101(Fe)/PS/vis system[118] |
AO7 + SO4·- / ·OH /· →···→
CO2 + H2O + ···
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
(楚宏宇, 王天宇, 王崇臣. 化学进展, 2022, 34 (12): 2700.).
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
(王茀学, 王崇臣. 环境科学研究, 2021, 34(12): 2924.).
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
(王茀学, 衣晓虹, 王崇臣, 邓积光. 催化学报, 2017, 38(12): 2141.).
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
(李渝航, 王鹏, 王崇臣, 刘艳彪. 无机化学学报, 2022, 38(12): 2342.).
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
(衣晓虹, 王崇臣. 化学进展, 2021, 33(03): 471.).
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
/
| 〈 |
|
〉 |