

Advanced Design of Block Copolymers for Nanolithography
Received date: 2023-03-09
Revised date: 2023-05-16
Online published: 2023-06-12
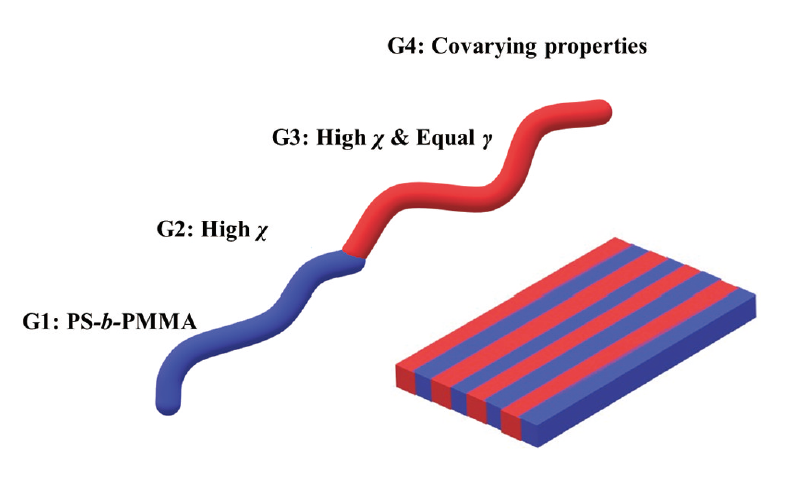
Directed self-assembly (DSA) of block copolymer (BCP) has been identified as the potential strategy for the next-generation semiconductor manufacturing. The typical representative of the first generation (G1) of block copolymer for nanolithography is polystyrene-block-polymethylmethacrylate (PS-b-PMMA). DSA of PS-b-PMMA enables limited half pitch (0.5L0) of 11 nm due to the low Flory-Huggins interaction parameter (χ). The second generation (G2) of BCP is developed with the feature of high χ. Solvent anneal or top-coat is employed for the G2 BCP to form the perpendicular lamellae orientation. Towards industry friendly thermal anneal, high χ BCP with equal surface energy (γ) is reported as the third generation (G3) BCP. Recently, based on Materials Genome Initiative (MGI) concept, optimized design of block copolymers with covarying properties (G4) for nanolithography is presented to meet specific application criteria. G4 BCP achieves not only high χ and equal γ, but also high throughput synthesis, 4~10 nm half pitch patterns, and controlled segregation strength. This review focuses on the advanced design of G3 and G4 BCP for nanolithography. Moreover, the challenges and opportunities are discussed for the further development of DSA of BCP.
1 Introduction
2 Highχblock copolymers with equalγ(G3)
2.1 A-b-B block copolymer
2.2 A-b-(B-r-C)block copolymer
2.3(A-r-B)-b-C block copolymer
2.4 A-b-B-b-C block copolymer
3 Block copolymers with covarying properties(G4)
4 Conclusion and outlook
Chen Leilei , Tao Yongxin , Hu Xin , Feng Hongbo , Zhu Ning , Guo Kai . Advanced Design of Block Copolymers for Nanolithography[J]. Progress in Chemistry, 2023 , 35(11) : 1613 -1624 . DOI: 10.7536/PC230304
图1 (a) 热退火密度倍增引导自组装DSA流程图[60];(b) 随着B嵌段体积分数的增加A-b-B二嵌段共聚物体相自组装结构示意图[62];(c) 对称二嵌段共聚物熔体的平均场相图[47]Fig.1 (a) Schematic of the fabrication process of the chemically patterned substrates and the directed assembly of PS-b-PMMA with density multiplication[60]. Copyright © 2013, American Chemical Society. (b) Schematic illustration of microstructures of diblock A-b-B on increasing the volume fraction of the B block[62]. Copyright © 2014, the Royal Society of Chemistry. (c) Mean-field phase diagram for conformational symmetric diblock melts[47]. Copyright © 1996, American Chemical Society |
图2 (a) PS-b-PLGA和PS-b-PDLLA的χ分别与温度倒数的关系图;(b) PS-b-PLGA引导自组装扫描电镜(SEM)图;(c) PS-b-PLGA热退火条件下2倍密度倍增DSA流程示意图[93]Fig.2 (a) Plots of χ against the inverse of temperature for PS-b-PLGA and PS-b-PDLLA; (b) top-down SEM images of DSA of PS-b-PLGA; (c) schematic illustration of the procedure used to create asymmetric chemical patterns and DSA of lamellae-forming PS-b-PLGA with 2×density multiplication under thermal annealing[93]. Copyright © 2018 American Chemical Society |
图5 PS-b-PPC的合成路线、图案化模板的创建以及热退火条件下5倍密度倍增DSA流程图及其SEM图[97]Fig.5 Synthesis of PS-b-PPC, schematic illustration of the procedure used to create prepatterned substrates and the DSA of lamellae forming PS-b-PPC system with 5 times density multiplication under thermal annealing, and the top-down SEM images showing DSA[97].Copyright © 2017 American Chemical Society |
图7 (a) PS-b-PMA、PMA-b-PS-b-PMA及其热退火引导自组装SEM图;(b) 动态储能模量(G')的温度依赖性;(c) χ值与温度倒数关系图[100]Fig.7 (a) Structure of PS-b-PMA and PMA-b-PS-b-PMA, and SEM of DSA; (b) temperature dependence of the dynamic storage modulus; (c) linear dependence of χ as a function of inverse TODT.[100]. Copyright © 2019 American Chemical Society |
图8 (a) 高χ近γ嵌段共聚物的结构设计;(b) χ值与温度倒数关系图;(c) PS-b-PGMA的TEM图[101]Fig.8 (a) Concept for designing a chemically tailored high-χ BCP with balanced surface affinities and increased strengths of segregation; (b) temperature dependences of the effective Flory-Huggins interaction parameter; (c) TEM images of PS-b-PGMA[101]. Copyright © 2019, the Royal Society of Chemistry |
图9 (a) PS-b-PI环氧化合成PS-b-(PI-r-PIxn);(b) 环氧化程度对嵌段共聚物相互作用参数(χ)的影响;(c) 环氧化程度对PI-r-PIxn表面能(γ)的影响[103]Fig.9 (a) Epoxidation of PS-PI to PS-b-(PI-r-PIxn); (b) effective interaction parameter (χ) affected by the degree of epoxidation; (c) effect of degree of epoxidation on the surface energy of PI-r-PIxn[103]. Copyright © 2012 American Chemical Society |
图10 (a) 利用烯-硫醇点击化学由PS-b-PB制备PS-b-P(B-r-Bthiol);(b) 巯基功能化反应程度(φ)对γ、L0和χ的影响;(c) LiNe flow DSA工艺流程图;(d) 未添加和添加0.1 wt% BHT的PS-b-P(B-r-BMEA)引导自组装SEM图[105]Fig.10 (a) Synthetic scheme of thiol-ene click chemistry to prepare PS-b-P(B-r-Bthiol) from PS-b-PB; (b) effects of degree of thiol functionalization (φ) on γ, L0, and χ; (c) schematic of the LiNe DSA process flow; (d) SEM of DSA of PS-b-P(B-r-BMEA) with and without 0.1 wt% BHT[105]. Copyright © 2022, Wiley-VCH GmbH, Weinheim |
图11 (a) 酯-酰胺交换反应修饰PS-b-PMMA;(b) 修饰前后对比(无序vs有序,低χ vs高χ);(c) 共聚物薄膜引导自组装SEM图[106]Fig.11 (a) Ester-amide exchange reaction of PS-b-PMMA with various amines; (b) comparison before and after ester-amide exchange reaction (disorder vs ordered, low χ vs high χ); (c) SEM of DSA of modified PS-b-PMMA[106]. Copyright © 2018, American Chemical Society |
图15 (a) 多功能嵌段共聚物A-b-(B-r-C)的设计思路:通过改变B和C获得高χ近γ 嵌段共聚物;(b) 从母体A-b-B’到系列A-b-(B-r-C)的合成示意图;(c) 基于巯基-环氧点击化学合成A-b-(B-r-C)[110]Fig.15 (a) Design principle for creating a series of BCPs with tunable χN and Δγ = 0 using an A-b-(B-r-C) polymer architecture. By varying the B and C groups, the architecture can form a BCP that has Δγ = 0 at the desired χ value; (b) schematic of the generation of a series of A-b-(B-r-C) polymers from the parent A-b-B'; (c) synthesis of A-b-(B-r-C) via thiol-epoxy click reactions[110]. Copyright © 2022, Springer Nature |
图16 (a) 多功能嵌段共聚物自组装薄膜具有增强的刻蚀对比度(使用SIS技术或引入含硅基团);(b) 多功能嵌段共聚物可以形成自身的中性层,免去传统DSA工艺中涂覆中性层的步骤[110]Fig.16 (a) Schematic of two distinct strategies for enhancing etch contrast of the self-assembled BCP film, using either SIS or silicon-containing thiols; (b) self-brushing DSA process flow leading to DSA with density multiplication[110]. Copyright © 2022, Springer Nature |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
( 李小欧, 顾雪松, 刘亚栋, 季生象. 应用化学, 2021, 38(9): 1105.)
|
| [21] |
( 陆新宇, 马彬泽, 罗皓, 齐欢, 李强, 伍广朋. 应用化学, 2021, 38(9): 1189.)
|
| [22] |
( 田昕, 赖翰文, 刘亚栋, 季生象. 应用化学, 2021, 38(9): 1199.)
|
| [23] |
|
| [24] |
( 顾雪松, 李小欧, 刘亚栋, 季生象. 应用化学, 2021, 38(9): 1091.)
|
| [25] |
( 胡晓华, 熊诗圣. 应用化学, 2021, 38(9): 1029.)
|
| [26] |
( 王倩倩, 吴立萍, 王菁, 王力元, 化学进展, 2017, 29(4): 435. )
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
( 陶永鑫, 陈蕾蕾, 刘一寰, 胡欣, 朱宁, 郭凯. 高分子学报, 2022, 53: 1445 )
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
/
| 〈 |
|
〉 |