

Research Progress in Synthesis of Titanium-Based Organic Framework Materials
Received date: 2023-04-13
Revised date: 2023-06-30
Online published: 2023-09-10
Supported by
National Natural Science Foundation of China(21908153)
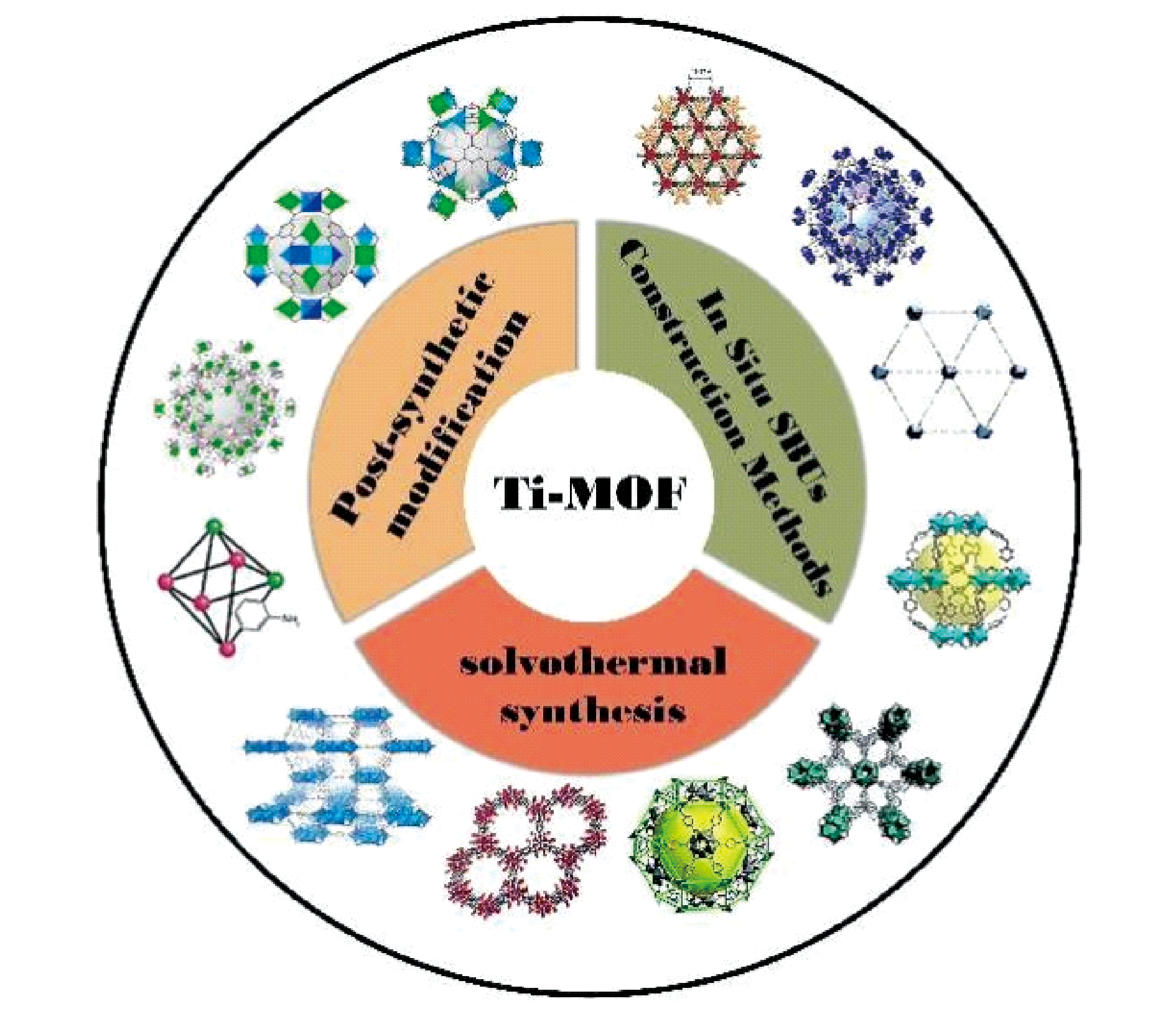
As a kind of metal-organic framework (MOF) with high valence, titanium-based metal-organic framework (Ti-MOF) has superior chemical stability, appealing photoresponsive properties, low toxicity and so on. However, due to the high reactivity of titanium sources, it brings certain challenges to the synthesis of materials. In this paper, the research progress of Ti-MOF synthesis in recent years is reviewed, and the solvothermal synthesis, post-synthetic modification and in situ SBUs construction methods are introduced in detail. The topological types and crystal structures formed are analyzed, and the synthesis rules of Ti-MOF and the advantages and disadvantages of various methods are summarized. It is pointed out that the control of the metal source and coordination environment is the most important strategy to obtain Ti-MOF, and the construction of Ti-MOF by in-situ formation of SBUs and heterometallic Ti/M-MOF are prospected.
1 Introduction
2 Synthesis of Ti-MOF
2.1 Solvothermal synthesis
2.2 Post-synthetic modification
2.3 In situ SBUs construction methods
3 Conclusion and outlook
Suhui Liu , Feifei Zhang , Xiaoqing Wang , Puxu Liu , Jiangfeng Yang . Research Progress in Synthesis of Titanium-Based Organic Framework Materials[J]. Progress in Chemistry, 2023 , 35(12) : 1752 -1763 . DOI: 10.7536/PC230415
| MOF | Ti-oxo-cluster | Bandgap energy (eV) | Application |
|---|---|---|---|
| MIL-125 | Ti8O8(OH)4(CO2)16 | 3.60 | Alcohol oxidation[18] |
| MIL-125-NH2 | Ti8O8(OH)4(CO2)16 | 2.60 | CO2 photoreduction, water splitting H2 production[21] |
| NTU-9 | TiO6 | 1.72 | Dye degradation[17] |
| PCN-22 | Ti7O6(CO2)12 | 1.93 | Alcohol oxidation[19] |
| MOF-901 | Ti6O6(CO2)6 | 2.65 | Polymerization[22] |
| ZSTU-3 | (Ti6O12)n | 2.20 | H2 production[23] |
| MUV-10 | Ti2Ca2(O)2(H2O)4(CO2)8 | 3.10 | H2 production[24] |
| MUV-101 | [TiM2(O) (O2C)6X3] (M=Mg, Fe, Co, Ni;) | — | Hydrolysis of nerve agent simulants[25] |
| FIR-125 | Ti8O8(OH)4(CO2)16 | 2.95 | CO2 photoreduction[26] |
表2 基于溶剂热法合成的Ti-MOFTable 2 Ti-MOF synthesized by solvothermal synthesis |
| Ti-MOF | Ti sources | Organic linker | Solvent environment | ref |
|---|---|---|---|---|
| MTM-1 | Ti(OiPr)4 | Isonicotinic acid | ACN | 32 |
| MIL-125 | Ti(OiPr)4 | Terephthalic acid | DMF、Dry CH3OH | 18 |
| NH2-MIL-125 | Ti(OiPr)4 | 2-Aminoterephthalic acid | DMF、Dry CH3OH | 21 |
| MIP-207 | Ti(OiPr)4 | Trimesic acid | Ac2O、CH3COOH | 33 |
| IEF | Ti(OiPr)4 | Squaric acid | IPA、CH3COOH | 34 |
| NTU-9 | Ti(OiPr)4 | 2,5-Dihydroxyterephthalic acid | CH3COOH | 17 |
| MIL-167 | Ti(OiPr)4 | 2,5-Dihydroxyterephthalic acid | DEF、CH3OH | 35 |
| COK-69 | Cp2TiCl2 | Trans-1,4-cyclohexanedicarboxybic acid | DMF、CH3COOH | 36 |
| Ti-MIL-101 | TiCl3 | Terephthalic acid | DMF、C2H5OH | 37,38 |
| MIL-177 | Ti(OiPr)4 | 3,3',5,5'-Tetracarboxydiphenylmethane | CH3OH | 39 |
| ZSTU-1 | Ti(OiPr)4 | 4,4',4″-Nitrilotribenzoic acid | DryDMF | 23 |
| ZSTU-2 | Ti(OiPr)4 | 1,3,5-Tris(4-Carboxyphenyl)benzene | DryDMF | 23 |
| ZSTU-3 | Ti(OiPr)4 | 4',4‴,4'''''-Nitrilotris([1,1'-biphenyl]-4-carboxylic acid) | DryDMF | 23 |
| Ti-(Ti-TBP) | TiCl4·2THF | Tetra(4-carboxyphenyl)porphine | DMF、CH3COOH | 40 |
| MUV-11 | Ti(OiPr)4 | Benzene-1,4-dihydroxamic acid | DMF、CH3COOH | 41 |
| ACM-1 | Ti(OiPr)4 | 4,4',4″,4‴-(1,9-dihydropyrene-1,3,6,8-tetrayl)tetrabenzoic acid | DEF-C6H5Cl(1∶1)、 C2H5COOH | 42 |
NoteTi(OiPr)4: Titanium tetraisopropanolate; Cp2TiCl2: Titanocene dichloride;DMF: N,N-Dimethylformamide;DEF: N,N-Diethylformamide; THF: Tetrahydrofuran; ACN: Acetonitrile; CH3OH:Methanol; CH3COOH: Acetic acid; Ac2O: Acetic anhydride; IPA: Isopropyl alcohol; C2H5OH: Ethanol; C2H5COOH: Propionic acid; C6H5Cl: Chlorobenzene |
表3 基于原位生成SBUs构筑法的Ti-MOFTable 3 Ti-MOF synthesized by in situ SBUs construction methods |
| Ti-MOF | Ti sources | Organic linker | Solvent environment | ref |
|---|---|---|---|---|
| PCN-22 Ti6O6(OiPr)6(abz)6 | Ti(iPrO)4 | 4-Aminobenzoic acid | IPA | 19 |
| PCN-22 | Ti6O6(OiPr)6(abz)6 | Tetrakis(4-carboxyphenyl)porphyrin | DEF、C6H5COOH、 | |
| DGIST-1 (Ti6O6(OiPr)6(t-BA)6) | Ti(iPrO)4 | 4-Aminobenzoic acid | IPA | 65 |
| DGIST-1 | Ti6O6(OiPr)6(t-BA)6 | Tetrakis(4-carboxyphenyl)porphyrin | DEF、C6H5COOH | |
| MIP-208 Ti8AF cluster | Ti(iPrO)4 | Ac2O | CH3COOH | 66 |
| MIP-208 | Ti8AF cluster | 5-Aminoisophthalic acid | Ac2O、C2H5COOH、CH3OH | |
| Ti3-BPBC (Ti6O6(OiPr)6(abz)6) | Ti(iPrO)4 | 4-Aminobenzoic acid | IPA | 67 |
| Ti3-BPBC | Ti6O6(OiPr)6(abz)6 | Biphenyl-4,4'-dicarboxylic acid | DMF、C2H5COOH | |
| MIL-100(Ti) Ti6O6(4-tbbz)6(OiPr)6 | Ti(iPrO)4 | 4-tert-butylbenzoic acid | IPA-THF (3∶1) | 68 |
| MIL-100(Ti) | Ti6 | Trimesic acid | ACN-THF(3∶1) |
NoteTi(OiPr)4: Titanium tetraisopropanolate;DMF: N,N-Dimethylformamide;DEF: N,N-Diethylformamide; THF: Tetrahydrofuran; ACN: Acetonitrile; CH3OH: Methanol; CH3COOH: Acetic acid; C2H5OH: Ethanol; C2H5COOH: Propionic acid; IPA: Isopropyl alcohol; Ac2O: Acetic anhydride; C6H5COOH: Benzoic acid |
表4 三种合成方法的对比Table 4 Comparison of three synthesis methods |
| Method | Advantage and disadvantage | Common synthesis condition | |
|---|---|---|---|
| Solvothermal synthesis | Ti-MOF | Ad: The available ligands are diverse, and the synthesized structures are rich with few restrictions. Dis: High reaction activity, easy hydrolysis, and the majority of synthesized sample powders. | Ti sources: organic Ti sources Solvent environment: organic solvent pH controller:acetic acid |
| Heterometallic Ti/M-MOF | Ad: Properly reduce the reactivity of Ti and synthesize materials with heterometallic advantages. Dis: The high polarizing power of Ti4+ prevents a direct reaction with other metals that would likely result in poor control over their distribution in the final material for the formation of segregated phases. | Ti sources:organic sources Solvent environment:organic solvent | |
| Post-synthetic modification | M-MOF transform into Ti/M-MOF by PSM | Ad: Capable of functionalizing the introduction of specific Ti ions into existing MOFs. Dis: 1. Titanium sources are prone to severe hydrolysis and are dangerous to operate. 2. Easy to be MOF@metal oxide rather than Ti/M-MOF | Ti sources:TiCl3、TiCl4 etc. Synthetic environment:Inert gas environment |
| Ti/M1-MOF transform into Ti/M2-MOF by PSM | Ad: Heterometallic Ti-MOF that cannot be obtained under high-temperature solvothermal conditions can be synthesized. Dis: Ti/M1-MOF is relatively rare, and not all bimetallic MOFs are suitable for this strategy. | ||
| In situ SBUs construction methods | Ad: It is another effective method to control the hydrolysis Condensation reaction reaction of Ti. Dis: The addition of reaction steps has made the stability of titanium clusters another factor limiting the reaction. | Ti sources:Ti6O6(OiPr)6(abz)6 Solvent environment:organic solvent | |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
/
| 〈 |
|
〉 |