

Metal-Organic Frameworks and Their Derivative Nano Anode Materials
Received date: 2023-05-04
Revised date: 2023-09-19
Online published: 2023-12-18
Anode is one of the important components for lithium ion battery. Many technical bottlenecks (such as lower ionic-electronic conductivity, huge volume effect and easy pulverization resulted from long-term charge/discharge process) prevent the development and large scale application of traditional anode materials. As a novel kind of advanced multi-functional materials, Metal-organic frameworks (MOFs) and their derivative materials behave enough pore structures promoting rapid migration of Li+ and electron, and high specific surface areas providing abundant active sites for electrochemical reaction. Importantly, tunable structure and chemical composition of the MOFs and their derivative materials can be further optimized by changing parameters of synthesis process, thereby markedly increases specific capacity and cycle stability of lithium ion batteries. Herein, the recent progress in the MOFs and their derivative materials used as anode for lithium ion batteries are reviewed systematically, and the relationships between their preparation methods, microstructures, morphologies and corresponding electrochemical properties are summarized detailly. The urgent problems and challenges of this class of anode materials for lithium ion batteries are also analyzed. On the basis of resonable choosing organic ligands and metal centers, some effective measures for improving performances of lithium storage are proposed by combining with the variability and particularity of structure of the MOFs and their derivative materials, and the feasible strategies for commercialization application are suggested. Finally, the perspective and future development in design and fabrication of the new types of nano porous anodes with high energy efficiencies in relation with the next generation lithium ion battery are further discussed.
1 Introduction
1.1 Conversion mechanism
1.2 Insertion/extraction mechanism
1.3 Absorption/desorption mechanism
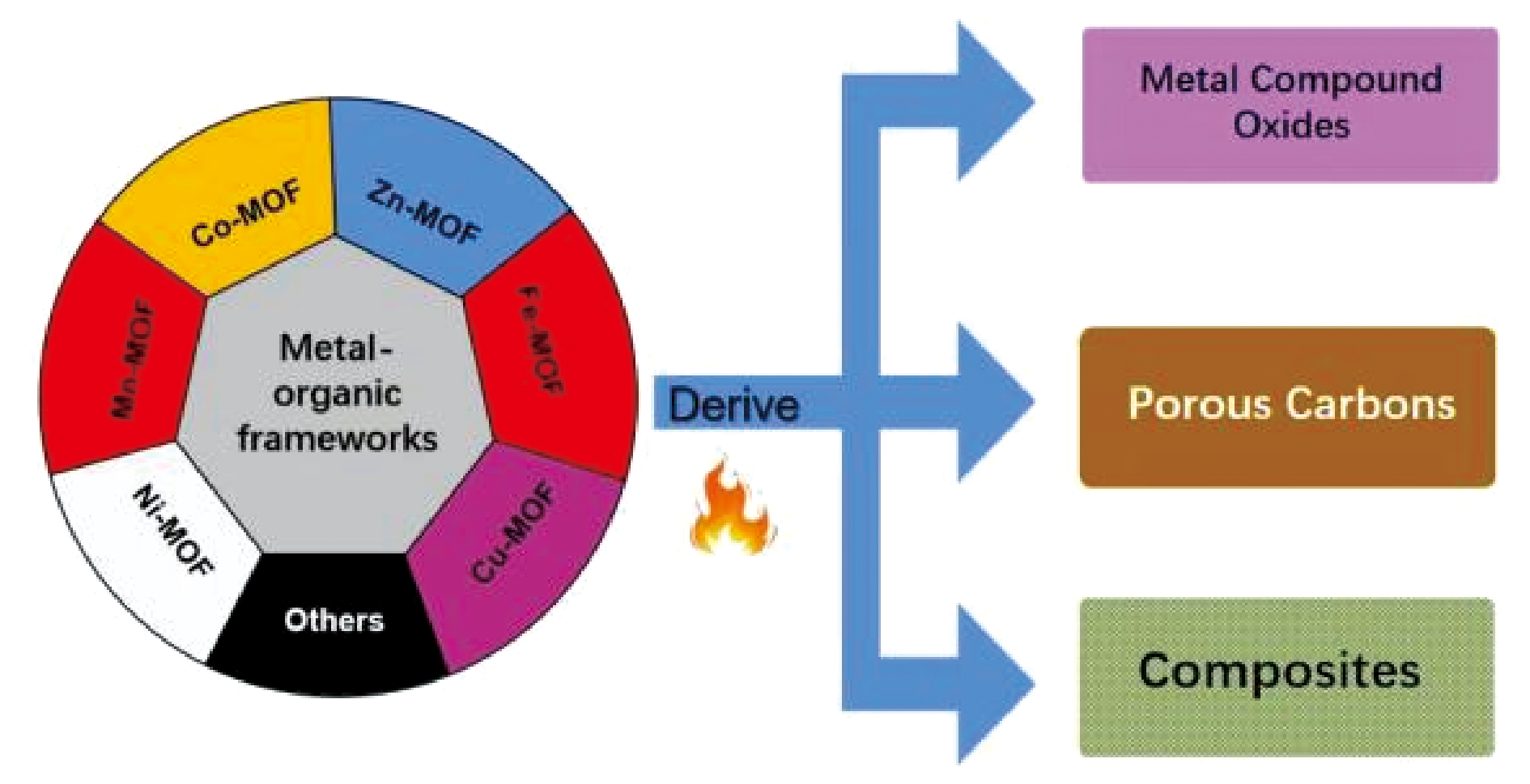
2 Pristine MOFs
2.1 Co-MOFs
2.2 Zn-MOFs
2.3 Mn-MOFs
2.4 Fe-MOFs
2.5 Ni-MOFs
2.6 Cu-MOFs
2.7 Sn-MOFs
2.8 Other metal-based MOFs
3 MOFs-derived metal compounds
3.1 Monometal oxides
3.2 Bimetal oxides
3.3 Other metal compounds
4 MOFs-derived porous carbon
5 MOFs-derived composites
5.1 MOFs/metal compounds
5.2 MOFs/carbon-based materials
5.3 metal oxide/Metal oxide
5.4 Metal oxide/carbon-based materials
5.5 Metal sulfide/carbon-based materials
5.6 Other metal compound/carbon-based materials
5.7 metal/metal oxide/carbon-based materials
6 Conclusion and outlook
Key words: lithium ion battery; anode; metal-organic frameworks; derivative materials
Haotian Ma , Rujin Tian , Zhongsheng Wen . Metal-Organic Frameworks and Their Derivative Nano Anode Materials[J]. Progress in Chemistry, 2023 , 35(12) : 1807 -1846 . DOI: 10.7536/PC230502
表1 MOFs本体负极材料及其电化学性能Table 1 Pristine MOFs as anode materials and their electrochemical performances |
| Materials | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity (mAh/g) | Initial discharge/ charge capacity (mAh/g) | Initial coulomb efficiency (%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|
| CoBTC-EtOH | 0.01-3.0 | 100/2000 | 100/500 | 856/473 | 1790.3/879 | 49.15 | 17.7 | 19 |
| S-Co-MOF | 0.01-3.0 | 100/500/1000 | 200/700/1000 | 1021/601/435 | 1964/1564 | 80.4 | 10.4 | 10 |
| H-Co-MOF | — | 100/2000 | 100/700 | 1345/828 | 2147/1432 | 66.7 | 49.9 | 20 |
| u-CoTDA | 0.01-3.0 | 100/1000/2000 | 100/300/400 | 946/790/548 | 1631/- | 75.2 | 52.6 | 22 |
| [Co1.5L(H2O)4]n | 0.01-3.0 | 50 | 50 | 431 | 1978/869 | — | — | 23 |
| Co2(DOBDC) | 0.01-3.0 | 100/500 | 100/200 | 878.5/526.1 | 1409/785 | 56 | — | 24 |
| Co-BDCN | 0.01-3.0 | 100 | 100 | 1132 | 1439/1015 | 70.54 | 24.5 | 25 |
| Co-BTC | 0.01-3.0 | 100 | 200 | 750 | 1739/622 | 36 | 18.5 | 26 |
| CoTPA | 0.005-2.8 | 60 | 100 | 700 | 1938/1004 | 51.8 | — | 27 |
| Co2(OH)2BDC | 0.02-3.0 | 50 | 100 | 650 | 1385/1005 | 72.8 | — | 28 |
| CoC6H2O5(H2O)2 | 0.05-3.0 | 100/1250 | 95/499 | 549.8/513.4 | -/- | — | — | 29 |
| Zn3(HCOOH)6 | 0.005-3.0 | 60 | 60 | 560 | 1344/693 | — | — | 30 |
| BMOF | — | 100 | 200 | 190 | -/- | — | 821 | 31 |
| Mn-LCP | 0.01-2.5 | 50 | 50 | 390 | 1807/- | — | — | 32 |
| CMPS-1 | 0.05-3.0 | 400/500 | 250/650 | 645.7/588.3 | 1631.8/- | — | — | 33 |
| Mn-BTC | 0.01-2.0 | 103/1030/2060 | 100/100/100 | 694/400/250 | 1717/694 | 40.4 | 23.8 | 34 |
| Mn-PBA | 0.01-3.0 | 200 | 100 | 295.7 | 1123.7/544.5 | 48.5 | 499.8 | 35 |
| Mn-1,4-BDC@200 | 0.01-3.0 | 100 | 100 | 974 | 1746/706.4 | 40.5 | 6.135 | 36 |
| Mn-UMOFNs | 0.01-3.0 | 100/1000 | 100/300 | 1187/818 | -/- | 57 | 32.65 | 38 |
| Fe-BTC | 0.01-3.0 | 100 | 100 | 1021.5 | 1765.5/683.2 | 38.7 | 1125 | 42 |
| MIL-88A | 0.01-3.0 | 10 | 4 | 40.5 | 140.5/5.3 | 4 | — | 43 |
| Fe-MIL-88B | 0.005-3.0 | 60 | 400 | 744.5 | 1507/949.9 | 63 | — | 44 |
| Fe-BDC@300 | 0.01-3.0 | 100 | 120 | 324.1 | 1330.6/- | 45 | ||
| Ni-UMOFNs | 0.01-3.0 | 100 | 100 | 546 | 1833/1226 | 67 | 15.04 | 38 |
| Ni-MOF | 0.01-3.0 | 100 | 100 | 620 | 1984/1369 | — | — | 46 |
| Ni-Me4bpz | 0.01-3.0 | 50 | 100 | 120 | 320/- | — | 67 | 14 |
| [Cu2(C8H4O4)4]n | 0.01-2.5 | 48 | 50 | 161 | 1492/194 | — | 747 | 48 |
| Cu3(BTC)2 | 0.05-3.0 | 96/191/383 | 50/50/50 | 740/644/474 | 1497/641 | — | 489.4 | 49 |
| [Cu2(cit)(H2O)2]n | 0.01-3.0 | 100/2000 | 500/500 | 608.5/321.5 | -/- | — | — | 50 |
| Sn-MOF | 0-3.0 | 20 | 200 | 610 | 1017/450 | — | 67.437 | 51 |
| Sn-MOF | 0.01-3.0 | 50 | 100 | 250 | -/- | — | 16.96 | 52 |
| Al-FumA MOFs | 0.01-3.0 | 37.5/37500 | 100/100 | 392/258 | 1509/899 | 45.5 | 260.1 | 53 |
| Pb-MOF | 0.01-3.0 | 100/500 | 500/500 | 489/380 | 1522/678 | 38 | 725 | 16 |
| Cd-MOF | 0.1-3.0 | 100 | 100 | 302 | 710/435 | — | 821 | 18 |
| Ti-MOF | 0.01-3.0 | 200/400 | 200/500 | 296/175.34 | 1590.24/- | — | 621 | 54 |
| Li/Ni-NTC | 0.01-3.0 | 100 | 80 | 482 | 1084/601 | — | — | 55 |
| Zn1.5Co1.5(HCO2)6 | 0.005-3.0 | 60 | 60 | 510 | 1344/693 | — | — | 30 |
图2 (a)CV曲线(0.1mV/s), (b) 潜在储锂位点 (Ⅰ 苯环; Ⅱ孔隙; Ⅲ层间空间), (c) 初始3次充放电曲线(0.2 A/g), (d) 倍率性能, (e)与其他负极在不同电流密度时的容量,(f) 0.2和0.5A/g时Ni-CAT NRs的循环性能[57]Fig. 2 (a) CV curves (0.1 mV/s), (b) potential lithium-storage sites (Ⅰ, benzene rings; Ⅱ, pores; Ⅲ, interlaminar space), (c) initial three charge and discharge plots (0.2 A/g), (d) rate behaviour, (e) comparison of capacities with other anodes at various current densities, and (f) cycling properties at 0.2 and 0.5 A/g of the Ni-CAT NRs[57] |
表2 MOFs衍生金属氧化物负极材料及其电化学性能Table 2 MOFs-derived metal oxides as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity (mAh/g) | Initial discharge/charge capacity (mAh/g) | Initial coulomb efficiency(%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| CuO | Cu(L-Phe)2 | 0.1~3.0 | 100/1000 | 200/500 | 505.3/116.7 | -/- | — | — | 62 |
| CuO | Cu-BTC | 0.05~3.0 | 100 | 100 | 470 | 1208/- | 40 | 49.6 | 63 |
| CuO | MOF-119 | 0.005~3.0 | 2000 | 40 | 210 | 1208/538 | — | — | 64 |
| CuO | Cu-BTC | 0.01~3.0 | 500/1000/2000 | 200/400/400 | 1062/615/423 | 1334.7/836.1 | — | 49.75 | 65 |
| Mn2O3 | Mn-MIL-100 | 0.1~3.0 | 200 | 100 | 755 | 1668/1003 | — | 40.45 | 66 |
| Mn2O3 | Mn-LCP | 0.01~3.0 | 1000 | 250 | 705 | 1158/852 | 74 | 15.34 | 67 |
| Mn2O3 | Mn-MOF | 0~3.0 | 400/1000 | 450/1200 | 1370/819.8 | -/- | — | — | 68 |
| Mn2O3 | Mn-BTC | 0.01~3.0 | 100 | 60 | 582 | 3404/1559 | 46 | 38.5 | 69 |
| Mn3O4 | Mn-MOF-74 | 0.01~3.0 | 200/2000 | 400/400 | 890.7/437.1 | 1078.9/625.1 | — | 80.5 | 70 |
| Co3O4 | [Co3(HCOO)6](DMF)4 | 0.01~3.0 | 50/100 | 50/100 | 965/730 | 1118/- | — | 5.3 | 71 |
| Co3O4 | Co-MOF | 0.01~3.0 | 1000/5000 | 350/600 | 628/412 | 1402/879 | 62.7 | 42 | 72 |
| Co3O4 | CoBDC MOF | 0.01~3.0 | 100/1000 | 160/200 | 1477/775 | 1392/961 | 69.09 | 133.74 | 73 |
| Co3O4 | Co-BTC | 0.00~3.0 | 100 | 60 | 886 | 2082/1061 | 51 | 10.44 | 74 |
| Co3O4 | Co-MOF | 0.01~3.0 | 100 | 50 | 1115 | 1608/1080 | — | 43 | 75 |
| Co3O4 | PBA | — | 300 | 50 | 1465 | 1557/- | — | 66.5 | 76 |
| Co3O4 | MOF-71 | 0.001~3.0 | 200 | 60 | 913 | 1286.1/879.5 | 68 | 59 | 77 |
| Co3O4 | Co2(NDC)2DMF2 | 0.01~3.0 | 200/2000 | 100/100 | 1058.9/348 | 1504.2/976.7 | 40 | 78 | |
| Co3O4-a | Co-MOF | 0.01~3.0 | 100 | 90 | 470.3 | 1325.5/1003.5 | 75.7 | 22.6 | 79 |
| Co3O4 | ZIF-67 | 0.01~3.0 | 100/100 | 100/60 | 1335/1265 | 1735/1083 | — | 45 | 81 |
| Co3O4 | Co-MOF | 0.01~3.0 | 100 | 100 | 1370 | 1324/1034 | — | 20.1 | 80 |
| α-Fe2O3 | MIL-88 | 0.01~3.0 | 200 | 50 | 911 | 1372/940 | 69 | 75 | 84 |
| α-Fe2O3 | Fe-MOF | 0.005~3.0 | 100 | 40 | 921.6 | 1487/1024 | — | — | 85 |
| Fe2O3-2 | MIL-53 | 0.005~3.0 | 100/1000 | 200/500 | 1176/744 | 1456/1048 | — | 93.1 | 86 |
| Fe2O3 | PB | 0.01~3.0 | 200 | 30 | 945 | -/- | — | 25.4 | 87 |
| NiO | MOF-C | 0.005~2.5 | 500/1000 | 100/100 | 748/410 | 2134/1303 | 61 | 36 | 88 |
| NiO | Ni-MOF | 0.01~3.0 | 15 | 100 | 380 | 900/480 | — | 24 | 89 |
| NiO | Ni-MOF | 0.0~3 | 200 | 100 | 760 | 1149/850 | 28.6 | 90 | |
| TiO2 | MIL-125 | 1.0~3.0 | 168/840/1680 | 500/500/500 | 166/106.5/71 | 168/- | — | 220 | 95 |
| GeO2 | Ge-MOF | 0.005~3.0 | 100 | 350 | 1393 | 2079/1315 | 63.2 | 12.9 | 96 |
| MnCo2O4 | Mn-Co-MOF | 0.01~3.0 | 100 | 100 | 929 | 1496/963 | 64 | 31.69 | 101 |
| Zn-NPs | ZIF-L | 0.01~3.0 | 100 | 100 | 143 | 1245.9/692.2 | 55.6 | 47.6 | 97 |
| ZnCo2O4 | ZnCo-8-hydroxyquinoline | 0.01~3.0 | 100/1500 | 50/25 | 1640.8/348.1 | 1710.2/1273.5 | 74.5 | 118 | 102 |
| Mn1.8Fe1.2O4 | Mn3[Fe(CN)6]2·nH2O | 0.01~3.0 | 200 | 60 | 827 | 2312/1337 | 57.8 | 124 | 103 |
| NixCo3-xO4 | Ni-Co-BTC | 0.005~3.0 | 100/1000/2000 | 100/300/300 | 1109.8/832/673 | 1619.2/1139.3 | 70 | 96.7 | 104 |
| ZnxCo3-xO4 | Zn-Co-ZIF | 0.01~3.0 | 100 | 50 | 990 | 1272/969 | 76.2 | 65.58 | 105 |
| CoFe2O4 | Co[Fe(CN)6]0.667 | 0.01~3.0 | — | — | — | 1352/1190 | 85.3 | 102.692 | 106 |
| NiFe2O4 | Ni2Fe(CN)6 | 0.01~3.0 | 914 | 200 | 1071 | 1245/1152 | — | 260.9 | 107 |
| Ni0.3Co2.7O4 | Co/Ni-MOF-74 | 0.005~3.0 | 100/2000/5000 | 200/500/500 | 1410/812/656 | 1737/1189 | — | 28.5 | 108 |
| Li4Ti5O12 | MIL-125 | 1.0~3.0 | 500 | 700 | 120.3 | 184.9/149.1 | 80.6 | — | 109 |
图3 NCNFs在Li+半电池中的电化学性能: (a) NCNFs-800在0.1 mV/s时的CV曲线, (b) NCNFs-800在0.1 A/g时的充放电曲线, (c) NCNFs在电流密度0.1~10 A/g时的倍率性能, (d) NCNFs-800在2 A/g时的循环性能[128]Fig. 3 The electrochemical performances of NCNFs in Li+ half cells. (a) CV curves of NCNFs-800 at 0.1 mV/s. (b)Discharge/charge profiles of NCNFs-800 at 0.1 A/g. (c) Cycling performances of NCNFs at 100 mA/g. (d) Rate capability of NCNFs at a current density from 0.1 to 10 A/g.(e) Cycling performance of NCNFs-800 at 2 A/g[128] |
表3 MOFs衍生多孔碳负极材料及其电化学性能Table 3 MOFs-derived porous carbon as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity (mAh/g) | Initial discharge/charge capacity (mAh/g) | Initial coulomb efficiency(%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| 3D porous carbon | Zn4O(BDC)3 | 0.01~3.0 | 100 | 100 | 1015 | 2983/1084 | — | 1880 | 114 |
| porous carbon | Zn-MOF | 0.01~3.0 | 74 | 50 | 2016 | -/2458 | — | 2587 | 121 |
| Porous carbon | Co-MOF | 0.01~3.0 | 100 | 49 | 549 | 3066/946 | — | 688 | 124 |
| Porous carbon | Cd-MOF | 0.01~3.0 | 300 | 300 | 1285 | 2486/1683 | 68 | 1796 | 125 |
| Porous CNFs | ZIF-8 | 0.01~3.0 | 100 | 200 | 520 | 570/390 | 68 | — | 126 |
| Nitrogen-modified carbon | Cu-MONFs | 0.01~3.0 | 500/5000 | 800/1000 | 853.1/440 | 1584.4/942.1 | 59.5 | 7.275 | 127 |
| N-C | Cu-MOF | 0.01~3.0 | 100/1000 | 100/1500 | 890/588 | 2037/1039 | 51 | — | 128 |
| N-P-C | Ni-ZIF | 0.01~3.0 | 100 | 200 | 570 | 1497/725 | 48.5 | 320.8 | 129 |
| N-NPC | Al-MOF | 0.01~3.0 | 1000 | 400 | 352 | 820/720 | 87.8 | 1244 | 130 |
| N-C-550 | Sr-MOF | 0.1~3.0 | 100 | 50 | 736.8 | 1043/675 | 64.71 | — | 131 |
| N-C-800 | ZIF-8 | 0.01~3.0 | 100/5000 | 50/1000 | 1147/785 | 3487/2037 | 58.4 | — | 134 |
表4 MOFs/多孔碳复合负极及其电化学性能Table 4 MOFs/ metal compound composites as anode materials and their electrochemical performances |
| Materials | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity(mAh/g) | Initial discharge/ charge capacity (mAh/g) | Initial coulomb efficiency(%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|
| Co-MOFs/CF | 0.01~3.0 | 50 | 100 | 445.1 | 1621.3/976.7 | 60.2 | 163.4 | 136 |
| MIL-101(Cr)/GO | 0.01~3.0 | 200 | 40 | 40.3 | 445.7/- | — | 3081 | 137 |
| Cu-MOF/RGO | 0.01~3.0 | 50 | 50 | 520 | 872.7/- | 45.8 | — | 138 |
| F-Co-MOF/RGO | 0.01~3.0 | 100/2000 | 50/550 | 1202/771.5 | 2464.2/1- | 73.45 | — | 140 |
| Fe-MOF/RGO(5) | 0.01~3.0 | 500 | 200 | 1010.3 | 2055.9/891.1 | 43.3 | — | 141 |
| MIL-53(Fe)@RGO | 0.01~3.0 | 100 | 100 | 550 | -/- | 42.3 | 240.9 | 142 |
| Co-BDC/CGr | 0.01~3.0 | 100/1000 | 100/400 | 1368/818 | 2566/- | 75.42 | 58.151 | 143 |
| MOF/RGOn | 0.01~3.0 | 100/1000 | 100/500 | 715/348 | 1677.5/732.6 | 43.7 | — | 145 |
图4 Fe2O3纳米管@Co3O4复合材料的合成示意图: (Ⅰ) MIL-88B纳米棒、Co2+和 2-methylimidazole (2-MIM)自组装成MIL-88B@ZIF-67复合材料; (Ⅱ) 经空气中热处理转变为Fe2O3纳米管@Co3O4复合材料[161]Fig. 4 Schematic illustration of the formation process of the Fe2O3 nanotubes@Co3O4 composite. (Ⅰ) Self-assembly of MIL-88B nanorods, Co2+ ions, and 2-methylimidazole (2-MIM) to a MIL-88B@ZIF-67 composite. (Ⅱ) Transformation to Fe2O3 nanotubes@Co3O4 composite through thermal treatment in air[161] |
表5 金属氧化物/金属氧化物复合负极材料及其电化学性能Table 5 Metal oxide/metallic oxide composites as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity (mAh/g) | Initial discharge/ charge capacity (mAh/g) | Initial coulomb efficiency (%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| 5Co3O4/CeO2 | Co-Ce-MOF | 0.01~3.0 | 100/500 | 100/300 | 1131.2/901.4 | 1090.1/873.6 | 77.6 | 19.265 | 152 |
| Fe2O3/SnO2 | Fe-MOF | 0.05~3.0 | 200 | 100 | 500 | 1751/904 | — | 43 | 153 |
| ZnO/NiO | Zn-Ni-MOF | 0.005~3.0 | 100/500 | 200/1000 | 1008.6/592.4 | 1221.7/769.2 | 62.9 | 21.34 | 154 |
| Co3O4/TiO2 | ZIF-67 | 0.01~3.0 | 500 | 200 | 642 | 662/535 | — | — | 155 |
| CuO/Cu2O | [Cu3(btc)2]n | 0.01~3.0 | 100 | 250 | 740 | 727/513 | — | 9 | 156 |
| CuO@NiO | Cu-Ni-BTC | 0.05~3.0 | 100 | 200 | 1061 | 1218/856 | — | 16.3 | 157 |
| Cr2O3@TiO2 | MIL-101(Cr) @TiO2 | 0.05~3.0 | 0.5C | 500 | 510 | 1138/- | — | 146 | 158 |
| CuO@TiO2 | HKUST-1/TiO2 | 0.01~3.0 | 100 | 200 | 692 | 1092/780 | 71.4 | 88.9 | 159 |
| Fe2O3-CuO | PB | 0.01~3.0 | 500 | 120 | 744 | 1070/795 | 74 | — | 160 |
| NiFe2O4@Fe2O3 | Fe2Ni MIL-88/ FeMIL-88 | 0.01~3.0 | 100 | 100 | 936.9 | 1400.9/989.1 | — | 39.2 | 163 |
| Fe2O3@NiCo2O4 | Co3[Fe(CN)6]2 @Ni3[Co(CN)6]2 | 0.01~3.0 | 100 | 100 | 1079.6 | 1311.4/902.7 | — | 12.72 | 164 |
| Co3O4-CoFe2O4-12 | MOF-74-FeCo-xy | 0.01~3.0 | 100/500 | 80/80 | 940/598 | 1328/918 | — | — | 165 |
| ZnO/ZnCo2O4 | ZnO@ZIF-8 NRAs | 0.01~3.0 | 1000/2000 | 200/250 | 870/1016 | 1299/987 | 76 | 20 | 166 |
| NiO/ZnCo2O4 | Ni(OH)2@ZIF-8 | 0.01~3.0 | 2000 | 100 | 1002 | -/- | — | 44 | 167 |
| ZnO/ZnFe2O4 | Zn3[Fe(CN)6]2 | 0.01~3.0 | 1000/2000 | 200/200 | 837/701 | 1892/1371 | 70 | 54.3 | 168 |
| ZnO/ZnFe2O4 | Prussian Blue | 0.01~3.0 | 1000/2000 | 500/100 | 804/497 | 1293/826 | 63.8 | 39.0 | 169 |
| ZnO/ZnFe2O4 | ZnFe PBA | 0~3.0 | 200 | 200 | 704 | 998.4/704.9 | 70.6 | — | 170 |
图5 In2O3/HPNC复合材料的形貌特征:(a)与(b) SEM图像;(c)与(d) TEM图像;(e) HRTEM图像(内部为SAED谱)(f~k) TEM暗场图像和相应元素分布[191]Fig. 5 Morphological features of In2O3/HPNC composite: (a) and (b) SEM images, (c) and (d) TEM images, (e) HRTEM images, and the inset is the SAED pattern, and (f~k)Dark-field TEM image and corresponding elemental mapping[191] |
表6 金属氧化物/碳基复合负极材料及其电化学性能Table 6 Metal oxide/carbon-based composites as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall capacity (mAh/g) | Initial discharge capacity/charge capacity (mAh/g) | Initial coulomb efficiency(%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Co3O4/C | ZIF-67 | 0.01~3.0 | 200 | 120 | 1100 | 1209/864 | 71.0 | 179.4 | 171 |
| ZnO@C | PPy@ZIF-8 | 0.01~3.0 | 250/1000/2000 | 500/500/1000 | 526/397/275 | 1106.2/665.8 | 60.2 | — | 174 |
| Co3O4/C | PPy@ZIF-67 | 0.01~3.0 | 250/1000/2000 | 500/500/1000 | 721/372/272 | 1112/645 | 58 | — | 174 |
| CuO/C | Cu-MOF | 0.01~3.0 | 100 | 200 | 789 | 1259/- | 76 | 131.7 | 175 |
| CuO/C | [Cu3(btc)2]n | 0.01~3.0 | 100 | 200 | 510.5 | 1150.9/450.4 | 46.2 | 16 | 176 |
| Mn3O4/C | Mn-PBA | 0.01~3.0 | 200 | 500 | 1032 | 1500/1205 | 80.3 | 137 | 177 |
| Mn3O4/C | MOF | 0.01~3.0 | 200/500/700 | 100/120/120 | 770/651/592 | 1186/722 | 60.8 | 8.0 | 178 |
| MnO/C | Mn-MOF | 0.01~3.0 | 50/500 | 150/500 | 884/648 | 1321.6/779.2 | 59 | 313 | 179 |
| MnO/C | Mn(PTA)-MOFs | 0~3.0 | 600/1000 | 100/200 | 804/800 | -/- | — | 309 | 180 |
| ZnO/C | Zn-BTC | 0.01~3.0 | 500 | 120 | 741 | 1205/715 | 59 | 198 | 181 |
| Fe3O4/C | Fe-MOFs | 0.01~3.0 | 100 | 100 | 861 | 1044.2/- | 82.4 | 27 | 186 |
| Fe3O4@C | Fe-MOF | 0.01~3.0 | 100 | 80 | 776.8 | 1714/1333 | 78 | 4.57 | 187 |
| SnO2@C | HKUST-1 | 0.001~3.0 | 100 | 200 | 880 | 2134/1208 | — | 474 | 189 |
| In2O3/C | MIL-68(In) | 0.01~3.0 | 100 | 150 | 720 | 1410/- | 43 | 152 | 190 |
| MWCNTs/Co3O4 | MWCNTs/ZIF-67 | 0.01~3.0 | 100 | 100 | 813 | 1171/812 | — | 62.9 | 192 |
| MWCNTs/ZnO | ZIF-8/MWCNTs | 0.01~3.0 | 200 | 100 | 419.8 | 1477/854 | — | 94.13 | 193 |
| NiO/CNTs-10 | Ni-MOF/CNTs | 0.005~3.0 | 100/2000 | 100/300 | 812/502 | 1100/- | — | 134.68 | 194 |
| CNT/Co3O4 | ZIF-67 | 0~3.0 | 1000/4000 | 200/200 | 782/577 | 1840/1281 | — | 93.9 | 195 |
| CFs@Co3O4 | CFs@ZIF-67 | 0.01~3.0 | 100 | 150 | 420 | 630/369.9 | 63 | 532.4 | 196 |
| 3DGN/CuO | Cu-BTC | 0.01~3.0 | 100 | 50 | 405 | 569/422 | 74 | — | 197 |
| NiO/GF | Ni-MOF/GF | 0.01~3.0 | 100 | 50 | 640 | 903/612 | 67.8 | 119 | 198 |
| Fe2O3/rGO | MIL-88-Fe/GO | 0.01~3.0 | 500/5000 | 200/500 | 846.9/610.3 | 1478/971 | — | — | 199 |
| RGO@Co3O4 | GO@ZIF | 0.01~3.0 | 100 | 100 | 974 | 1451/- | 70 | 198.54 | 200 |
| RGO/NiO | GO/Ni-MOFs | 0~3.0 | 100 | 200 | 440 | 681/678 | 99.49 | — | 201 |
| MnO/C-N-500 | Mn-PBI | 0.01~3.0 | 300 | 100 | 1085 | 1507/1143 | 75.8 | 146.4 | 203 |
| NiO@N-C | Ni-NTA | 0~3.0 | 50/4000 | 300/1200 | 921/450 | 1220/1009 | 82.3 | — | 204 |
| Fe2O3@N-C | Fe-ZIF | 0.01~3.0 | 100/1000 | 50/100 | 1573/1142 | 1696/1368 | 80.7 | 27.1 | 206 |
| NCW@Fe3O4/NC | NCW@Fe-ZIFs | 0.01~3.0 | 100/1000 | 170/600 | 1963/1741 | 2867/1585 | 55.3 | 52.7 | 208 |
| Co3O4/N-C | Co-TATB MOFs | 0.0~3.0 | 1000 | 200 | 620 | 1062/- | 75.0 | — | 209 |
| Co3O4/N-C | N-rich Co-MOF | 0.01~3.0 | 1000 | 500 | 612 | 1210/613 | 51 | 21.5 | 210 |
| Co3O4/N-PC | ZIF-67 | 0.05~3.0 | 100 | 100 | 892 | 1730/1321 | 76.4 | 97 | 211 |
| Co3O4@N-C | Co-MOF | 0.1~3.0 | 500 | 300 | 795 | 1385/1055 | 76 | 30.16 | 212 |
| MS-Co3O4@PC | Co-MOFs | 0.01~3.0 | 100/1000 | 60/500 | 1701/601 | 1470/1188 | 80.8 | 22.1 | 213 |
| CoO-NCNTs | 2D-MOFs | 0.01~3.0 | 500 | 2000 | 583 | 1156/945 | 81.7 | 86.26 | 214 |
| Co3O4@NGN | ZIF-67@NGA | 0.01~3.0 | 200/1000 | 100/400 | 955/676 | 976/865 | 52.3 | 50 | 215 |
| ZnO@C(30) | ZnO@ZIF-8 | 0.02~3.0 | 100/1000 | 100/300 | 539/498 | 1065/664 | 62 | — | 216 |
| Co-doped ZnO@C | Co-MOF-5s | 0.01~3.0 | 100 | 50 | 725 | 903/- | — | — | 217 |
| Ti-doped-CoO@C | Co-Ti-MOF | 0.01~3.0 | 200 | 150 | 1180 | 1749/830.7 | — | 241.4 | 218 |
| C/CoTiO3 | Co-MOF | 0.005~3.0 | 100/2000 | 100/1400 | 630/610 | -/- | 75.8 | 62.2 | 220 |
| NiFe2O4/N-C | NiFe-MOF | 0.005~3.0 | 100/500 | 50/50 | 760/610 | 1193/773 | 64.8 | 146.2 | 221 |
| NiFe2O4/CNTs | Fe2Ni MIL-88 | 0.01~3.0 | 100/2000 | 100/100 | 624.6/250 | 1348/1030 | 76.4 | 70.73 | 222 |
| CNTs@ZnCo2O4 | ZIF-8 | 0.01~3.0 | 100 | 100 | 750 | 682.7/435.6 | — | 78 | 167 |
| CoFe2O4/GNS | Co-Fe-BTC | 0.01~3.0 | 100 | 100 | 1061.7 | 1413/1058 | — | 24.5 | 225 |
| Zn0.5MnO@C | Zn-Mn-BTC | — | 100/5000 | 200 /500 | 1050/408 | 1565.9/954.6 | — | 30.8 | 226 |
| MnO-doped Fe3O4@C | Mn-doped MIL-53 (Fe) | 0.01~3.0 | 200 | 200 | 1297.5 | 1281.4/938.6 | 73.3 | 63.3 | 227 |
| Co3O4/NiO/C | CoNi-MOFs | 0.01~3.0 | 1000 | 1000 | 776 | 1522/907 | — | 136 | 229 |
| Fe-Mn-O/C | Fe/Mn-MOF-74 | 0.0~3.0 | 100 | 200 | 1294 | 1333/837 | — | 158.2 | 230 |
| Co3O4@CuO @GQDs | Co-Cu-BTC | 0.005~3.0 | 100 | 200 | 1054 | 1352/816 | 60 | 36 | 231 |
| ZnO/ZnFe2O4/C | MOF-5 | 0.005~3.0 | 500/2000 | 100/100 | 1390/988 | 1385/1047 | 75.6 | 140 | 232 |
| ZnO/Ni3ZnC0.7/C | Zn-MOF/Ni | 0.01~3.0 | 500 | 750 | 1002 | 1743/1015 | 58.2 | 112 | 233 |
| ZnO/ZnFe2O4@C | ZFC | 0.01~3.0 | 1000 | 500 | 718 | 1392/1059 | 76.1 | 80 | 234 |
| C@ZnO/ZnCo2O4/CuCo2O4 | Co-Cu-ZIF-8 | 0.01~3.0 | 300/3000/10000 | 500/500/500 | 1742/1009/664 | 2430/1967 | 80.1 | 549.7 | 235 |
图6 (a)合成过程示意图, (b) SEM图像, (c) 高分辨TEM图像,(d) 合成的MoS2⊂C的STEM-EDS分布 ((c)的内部为SAED谱)[261]Fig. 6 (a) Schematic illustration of the fabrication process, (b) SEM image, (c) high-resolution TEM image and (d) STEM-EDS mapping of the as-synthesized MoS2⊂C hybrids (inset of (c) showing the corresponding SAED pattern)[261] |
表7 金属硫化物/碳基复合负极材料及其电化学性能Table 7 Metal sulfide/carbon-based composites as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall Capacity (mAh/g) | Initial discharge capacity/charge capacity (mAh/g) | Initial coulomb efficiency (%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| H-Co9S8@C | Co-MOF-74 | 0.01~3.0 | 100/500 | 250/50 | 900.5/655 | 1119.5/867.3 | 77.4 | 127.1 | 238 |
| Co9S8@NMCN | ZIF-67 | 0.01~3.0 | 100 | 80 | 988 | 1705/1125 | 66 | 76.9 | 239 |
| Co9S8/N-C | ZIF-67 | 0.01~3.0 | 544 | 400 | 784 | 1260/900 | 71.48 | 125.9 | 240 |
| Co9S8/S-NC | ZIF-67 | 0~3.0 | 1000 | 300 | 500 | 879.7/529.3 | 60.17 | 150.5 | 242 |
| Co9S8/NSC | Sulfonate-based Co-MOF | 0.01~3.0 | 100/2000 | 200/1000 | 1179/789 | 1816.9/862 | 47.5 | 228.1 | 243 |
| CoS@PCP/ CNTs-600 | ZIF-67 | 0.01~3.0 | 200 | 100 | 1668 | 2083/1246 | — | 101.5 | 245 |
| NC/CoS2-650 | ZIF-67 | 0.1~3.0 | 100/2500 | 50/50 | 560/410 | 1100/ | — | — | 246 |
| CoS2-N-C/3DGN | Co-MOF | 0.01~3.0 | 100 | 100 | 409.5 | 833.5/666.3 | 79.9 | — | 247 |
| CoS2/NSCNHF | ZIF8@ZIF67 | 0.01~3.0 | 100/1000 | 100/200 | 845/549.9 | 1155.6/739.6 | 64 | 234.25 | 248 |
| Co3S4/MNCNT | ZIF-67/MWCNT | 0.01~3.0 | 200/2000 | 50/500 | 1281.2/976.5 | 1644.2/1055 | 64.17 | 112 | 249 |
| Co1-xS/C | Co-BTC | 0.01~3.0 | 200/1000 | 100/700 | 791/667 | 1290/932 | 72 | 124 | 250 |
| FeS/C | Fe-MOFs | 0.01~3.0 | 100 | 150 | 830 | 1702/972 | 57 | 0.015 | 251 |
| FeS2@POC | Fe-MOFs | 0.01~3.0 | 100/2000 | 100/200 | 1074/607 | 1394/1115 | 80 | 44.2 | 252 |
| C@Fe7S8 | MIL-88 | 0.01~3.0 | 500 | 170 | 1148 | 1072/761 | 71 | 277 | 253 |
| ZnS/PC | MOF-5 | 0.01-2.5 | 100 | 300 | 438 | 1220/- | — | 296.8 | 254 |
| ZnS@NC | ZIF-8 | 0.01~3.0 | 200/500 | 150/450 | 521.8/853 | 1284.9/840.6 | 34.6 | 191.45 | 255 |
| α-MnS/SCMFs/Cu | Mn-MOF | 0.01~3.0 | 200/1500 | 300/1000 | 1383/601 | 1115/- | 69 | 109 | 258 |
| MoS2@NC-2 | ZIF-8 | 0.005~3.0 | 100 | 50 | 715 | 1864/- | 50 | — | 259 |
| ZnCoS@Co9S8/NC | ZIF-67@ZIF-8/ ZIF-67 | 0.01~3.0 | 500/2000 | 500/400 | 1814/1095 | 2895/2182 | — | 270.46 | 263 |
表8 其他金属化合物/碳基复合负极材料及其电化学性能Table 8 Other metal compounds/carbon-based composites as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall Capacity (mAh/g) | Initial discharge capacity/charge capacity (mAh/g) | Initial coulomb efficiency (%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| CoSe@PCP | ZIF-67 | 0.005~3.0 | 200/1000 | 100/500 | 675/708.2 | 902/603 | 73.5 | 76.94 | 264 |
| 3DG/Fe7Se8@C | 3DG/MOF | 0.01~3.0 | 200/1000 | 120/250 | 884.1/815.2 | — | — | — | 265 |
| Ni-Co-Se/C-600 | Ni-Co-BTC | 0.01~3.0 | 1000/3000 | 500/900 | 1514/852 | 821/634 | 77 | 127 | 266 |
| Bi2Se3@C | Bi-MOF | 0.01~3.0 | 200/1000 | 200/5000 | 637/543 | -/642 | 68 | 76 | 267 |
| ZnSe/NC-300 | ZIF-8 | 0.01~3.0 | 100 | 500 | 724.4 | 906.66/547.48 | 60.3 | 93.926 | 268 |
| Ni2P/NC | MOF-Ni | 0.01~3.0 | 500 | 800 | 450.4 | 1240.5/649.5 | — | 34.5 | 269 |
| CoP/C | ZIF-67 | 0.01~3.0 | 500 | 1000 | 523 | 1522/1110 | 72.9 | 67.2 | 270 |
| CoxP-NC-800 | ZIF-67 | 0.01~3.0 | 100/1000 | 100/1800 | 1224/400 | 2450/1469 | — | 326.5 | 271 |
| MoC@C-700 | Mo3(BTC)2 | 0.01~3.0 | 1000 | 500 | 509.8 | 990.8/674.3 | 65.3 | 187 | 272 |
图7 (A) SnZCw前驱体和(B)不同放大倍数的SnZCw的SEM图像;SnZCw的(C) TEM图像、(D) HRTEM图像和(E) 选区电子衍射; (F) 不同放大倍数的SnZCd TEM图像[276]Fig. 7 SEM images of (A) the precursor of SnZCw, and (B) SnZCw at different magnifications; (C) TEM image, (D) HRTEM image, and (E) the SAED pattern of SnZCw; (F) TEM images at different magnifications of SnZCd[276] |
表9 金属/金属氧化物/碳基复合负极材料及其电化学性能Table 9 Metal/metal oxide/carbon-based composites materials as anode materials and their electrochemical performances |
| Materials | Template/precursor | Voltage range (V) | Current density (mA/g) | Cycle number | Overall Capacity (mAh/g) | Initial discharge capacity/charge capacity (mAh/g) | Initial coulomb efficiency (%) | Specific surface area (m2/g) | ref |
|---|---|---|---|---|---|---|---|---|---|
| Co3O4/Co/C | ZIF-67 | 0.01~3.0 | 100/2000 | 60/600 | 801/505 | 1158/867 | 75 | 183.9 | 274 |
| Co/Co3O4@N-C-700 | NUM-6 | 0.01~3.0 | 100/1000 | 100/100 | 903/774 | 1535/830 | — | 250.4 | 275 |
| Sn/C-ZnO | ZIF-8 | 0.01~3.0 | 100 | 50 | 515.6 | 1118.7/- | 64.2 | 32.9 | 276 |
| SnO2/Co@C | CoSnO3 @MOF | 0.01-2.5 | 200/5000 | 100/1800 | 800/400 | 1300/857 | 66 | 84 | 277 |
| NiO/Ni/Graphene | Ni-MOFs | 0.005~3.0 | 2000 | 250 | 1180 | 1759/1144 | — | 104 | 278 |
| Ni@ZnO/CNF | Ni@ZIF-8 | 0.01~3.0 | 100 | 100 | 1051 | 1547/1100 | 71 | 38.6 | 279 |
表10 MOFs及其衍生负极材料的优点和缺点Table 10 Advantages and disadvantages of MOFs and their derivative anode materials |
| Materials | Advantages | Disadvantages |
|---|---|---|
| MOFs | abundant active sites large specific surface area high porosity adjustable composition, morphology and structure low cost | low conductivity poor structure stability fast capacity decay |
| Porous carbon | large specific surface area high porosity simple synthesis method and mild synthesis condition high thermal stability no subsequent complex physical or chemical activation | insufficient capacity uncontrolled structure evolution inherent structure properties determined from micropore |
| Single metal oxide | simple synthesis method controllable synthesis path Large specific surface area high porosity controlled structure and composition | limited species lower conductivity easy volume expansion and pulverization lower rate capability and cycle performance |
| Double metal oxides | stronger synergistic effect between different metal elements abundant redox sites faster reaction kinetics and activity | lower conductivity easy volume expansion and pulverization lower rate capability and cycle performance |
| Other metal compounds | wide variety good mechanical and thermodynamic stability higher theoretical capacity | complicated synthesis process higher cost lower reaction kinetics under high load lower utilization |
| MOFs/ Metal oxide | abundant active sites high porosity alleviated volume expansion and pulverization | lower conductivity |
| MOFs/C | high porosity better structure stability higher conductivity | limited capacity |
| Metal oxide/Metal oxide | stronger synergistic effect between different metal elements improved electrode integrity alleviated volume effect | lower conductivity easy volume expansion and pulverization limited capacity and cycle stability |
| Metal oxide/C | higher conductivity better structure stability faster electron transportation stronger synergistic effect | complicated synthesis method uncontrolled synthesis condition |
| Metal sulfide/C | higher conductivity better structure stability stronger synergistic effect | slower reaction kinetics faster capacity decay |
| Other metal compounds/C | abundant active sites higher utilization rate of active substances faster electron transportation stronger synergistic effect | insufficient performance under high load ambiguous reaction mechanism |
| Metal/Metal oxide/C | higher conductivity better structure stability faster electron transportation stronger synergistic effect | complicated synthesis process ambiguous reaction mechanism |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
(李振杰, 钟杜, 张洁, 陈金伟, 王刚, 王瑞林. 化学进展, 2019, 31: 201.).
|
| [6] |
(赵云, 亢玉琼, 金玉红, 王莉, 田光宇, 何向明. 化学进展, 2019, 31: 613.).
|
| [7] |
|
| [8] |
C,
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
(邢锦娟, 史发年, 薛冬峰. 化学研究, 2020, 31(2): 95.).
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
(杜婕. 西安科技大学硕士论文, 2017.).
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
(胡小诗. 华东师范大学博士论文, 2018.).
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
(姚远, 侯宏英, 刘显茜, 田川, 孟堃, 兰建, 徐加雷, 冯蒙蒙. 人工晶体学报, 2020, 49(7): 1242.).
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
(陈川, 钱森, 丁一, 姚天浩, 郭经红, 王红康. 功能材料, 2020, 51(10): 10116.).
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
(曹娜, 杜慧玲, 王金磊, 马武祥, 马万里, 田超. 硅酸盐学报, 2018, 46(12): 1748.).
|
| [137] |
|
| [138] |
(高国梁, 王德宇, 曾群, 沈彩. 华南师范大学学报(自然科学版), 2018, 50(2): 30.).
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
(唐波. 北京化工大学硕士论文, 2017.).
|
| [180] |
SunD,
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
|
| [196] |
|
| [197] |
JiD,
|
| [198] |
|
| [199] |
|
| [200] |
|
| [201] |
|
| [202] |
|
| [203] |
|
| [204] |
|
| [205] |
|
| [206] |
(郑方才. 中国科学技术大学博士论文, 2015.).
|
| [207] |
|
| [208] |
|
| [209] |
|
| [210] |
|
| [211] |
|
| [212] |
|
| [213] |
|
| [214] |
|
| [215] |
|
| [216] |
|
| [217] |
|
| [218] |
|
| [219] |
|
| [220] |
|
| [221] |
|
| [222] |
|
| [223] |
|
| [224] |
(蔡默超, 蔡森荣, 郑明森, 董全峰. 电化学, 2014, 20(2): 101.).
|
| [225] |
|
| [226] |
WangD,
|
| [227] |
|
| [228] |
|
| [229] |
|
| [230] |
|
| [231] |
|
| [232] |
|
| [233] |
|
| [234] |
|
| [235] |
|
| [236] |
|
| [237] |
|
| [238] |
|
| [239] |
|
| [240] |
|
| [241] |
|
| [242] |
|
| [243] |
|
| [244] |
|
| [245] |
|
| [246] |
|
| [247] |
|
| [248] |
|
| [249] |
|
| [250] |
|
| [251] |
|
| [252] |
|
| [253] |
|
| [254] |
|
| [255] |
|
| [256] |
|
| [257] |
|
| [258] |
|
| [259] |
|
| [260] |
|
| [261] |
|
| [262] |
|
| [263] |
|
| [264] |
|
| [265] |
|
| [266] |
|
| [267] |
|
| [268] |
|
| [269] |
|
| [270] |
|
| [271] |
|
| [272] |
|
| [273] |
(闫浩然, 邱帆, 汪楷丽, 张大鹏, 陈君华, 牛斐洱. 塑料科技, 2020, 48(11): 7.).
|
| [274] |
|
| [275] |
|
| [276] |
|
| [277] |
|
| [278] |
|
| [279] |
|
/
| 〈 |
|
〉 |