

Nanomaterials-Mediated Autophagy-Based Cancer Treatment
Received date: 2023-03-30
Revised date: 2023-05-21
Online published: 2023-09-11
With the rapid development of nanotechnology, nanomaterials have been widely used in the field of cancer treatment. It is well known that autophagy, as a process that maintains cellular homeostasis, plays a dual role in promoting survival and death in cancer development. The level of autophagy in cancer cells is significantly higher than that in normal cells, resulting in various treatment strategies being ineffective. Synergistic treatment of cancer by perturbing autophagy has become a viable option, but traditional autophagy perturbing agents such as chloroquine may lead to certain other side effects. In addition, it has been demonstrated that nanomaterials can be used as a novel autophagy perturber, but the mechanism by which nanomaterials interfere with autophagy needs to be more deeply understood. This review provides an overview of the dual relationship between cancer and autophagy, and highlights the mechanisms by which various nanomaterials induce cancer cell death or apoptosis by perturbing autophagy, or enhance the sensitivity of cancer cells to conventional cancer therapy by perturbing autophagy, and the mechanisms by which they modulate autophagy.
1 Introduction
2 Cancer and autophagy
2.1 Autophagy inhibits the occurrence of cancer
2.2 Autophagy promotes cancer development
3 Effect of nanomaterials on autophagy
4 Nanomaterials treat cancer by perturbing autophagy
4.1 Metallic nanomaterials
4.2 Oxide nanomaterials
4.3 Carbon based nanomaterials
4.4 Other Nanomaterials
5 Mechanisms of autophagy perturbed by nanomaterials
5.1 Oxidation stress
5.2 Perturbation of autophagy-related signaling pathways
5.3 Lysosomal dysfunction
6 Conclusion
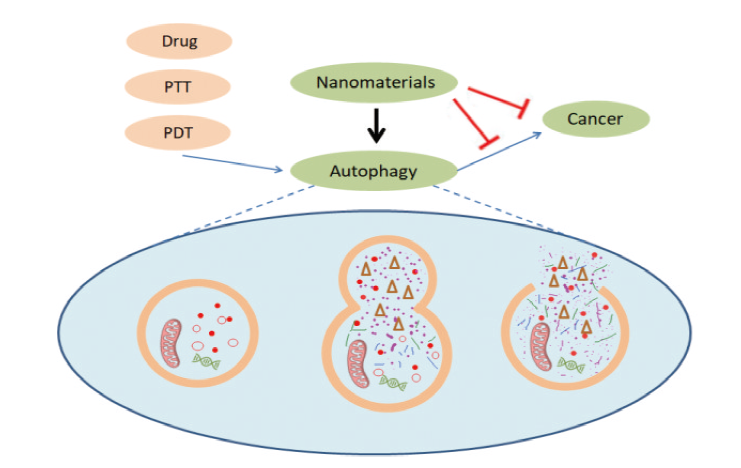
Key words: nanomaterials; cancer; autophagy; drug resistance
Jin Weitao , Yang Ting , Jia Jimei , Zhou Xiaofei . Nanomaterials-Mediated Autophagy-Based Cancer Treatment[J]. Progress in Chemistry, 2023 , 35(11) : 1655 -1673 . DOI: 10.7536/PC230330
表1 纳米材料通过调控自噬治疗癌症Table 1 Nanomaterials treat cancer by regulating autophagy |
| NMs | Size (nm) | shape or dispersity | Coating | Drug or therapies | Model cells | Mechanism | Autophagy | Effect | ref |
|---|---|---|---|---|---|---|---|---|---|
| Au NPs | 20 | sphere | PEG | immunotherapy | Hepa1-6 cells; RAW 264.7 cells | Lysosome alkalization; membrane permeabilization | Inhibition | Increased sensitivity | 79 |
| Au NPs | 30~60 | Peanut | Bare | — | SKOV-3 cell | ROS upregulation | Inhibition | Apoptosis | 78 |
| Ag NPs | 66.92 | sphere | Bare | — | PC-3 cell | lysosome injury; cell hypoxia | Inhibition | Cell death | 93 |
| Fe3O4-Au NPs | 15~25 | sphere | — | DOX | HepG2 cells | Enhancing autophagosome formation | Induction | Reduce drug resistance | 80 |
| Ag NPs | 59 | sphere | Bare | — | HT-29 cells | JNK activation and eIF2α phosphorylation | Induction | Apoptosis | 97 |
| Fe@Au NPs | — | core-shell structure | — | — | OECM1 cell | Mitochondria damage | Inhibition | Cell death | 73 |
| Ag NPs | 26.5 | sphere | PVP | — | HeLa cell | PtdIns3K-dependent | Induction | Ehances the anticancer activity | 90 |
| Fe3O4 NPs | 36 | sphere | PEG | PTX | U251 cell | ROS upregulation | Induction | Reduce drug resistance | 98 |
| Ag NPs | 13 | sphere | — | — | A549 cell | ROS upregulation | Induction | Apoptosis | 83 |
| Ag NPs | 8 | sphere | protein | Cisplatin | OS cell; HCC cell | MAPK pathways | Induction | Reduce drug resistance | 89 |
| Fe2O3 NPs | 10 | sphere | DMSA | — | SK-Hep-1 cell | ROS upregulation; MAPK pathways | Induction | Cell death | 99 |
| CuO NPs | 10 | sphere | — | — | MCF7 cell | ROS dependent | Induction | Growth inhibition | 100 |
| ZnO NPs | 21 | — | — | Sorafenib | Huh 7 cell | Promoting p53 Gene | Induction | Apoptosis | 101 |
| ZnO NPs | 63 | — | — | — | MCF7 cell | — | Inhibition | Apoptosis | 102 |
| SiO2 NPs | 86 | sphere | HCT-116 cells | ER stress | Induction | Cell survival | 103 | ||
| Ag NPs | 15.38 | sphere | PVP | Radiotherapy | U251 cells | ROS upregulation | Induction | Increased sensitivity | 88 |
| IONPs | 37 | — | PEG | — | U251 cells | Beclin 1/ATG 5 pathways | Induction | Ferroptosis | 30 |
| Gd2O3 NPs | — | — | — | Cisplatin | HeLa cells | — | Inhibition | Reduce drug resistance | 104 |
| SiO2 NPs | 125 | sphere | — | Propranolol | HemSCs cells | ER stress | Induction | Cell death | 105 |
| ZnO NPs | 20 | — | — | — | SKOV3 cells | ROS upregulation | Induction | Apoptosis | 106 |
| SiO2 NPs | 198 | sphere | PDA; PEG | DOX | MCF7 cells | AKT-mTOR-p70S6K pathway | Induction | Cell death | 40 |
| ND | 191 | — | — | Hypoxia | HeLa cells; MCF7 cells | — | Inhibition | Apoptosis | 107 |
| ZnO NPs | — | — | — | Cisplatin | SGC7901 cells;BGC823 cells | — | Inhibition | Reduce drug resistance | 108 |
| GO | 450 | sheet | DMSO | Cisplatin | Skov-3 cells | — | Induction | Cell death | 109 |
| DWCNTs | — | Tube | — | — | DHD/K12/Trb cell line | Intracellular acidification | Induction | Cell death | 110 |
| Se NPs | 70 | Amorphous solid | — | Astragalus Polysaccharides | MCF7 cells | ROS upregulation and Mitochondria damage | Inhibition | Apoptosis | 111 |
| BPQDs | 140 | Monodisperse | Platelet membrane | Hederagenin | MCF7 cells; RAW 264.7 cells | ROS upregulation and Mitochondria damage | Induction | Apoptosis | 112 |
| Co3O4 NPs | 200 | sphere | — | Photothermal therapy | U-87 MG cells | Llysosomal function damage | Blockage of autophagic flux | Cell death | 113 |
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|
| [124] |
|
| [125] |
Ibrahim Abdel Aziz I,
|
| [126] |
|
| [127] |
|
| [128] |
|
| [129] |
|
| [130] |
|
| [131] |
|
| [132] |
|
| [133] |
|
| [134] |
|
| [135] |
|
| [136] |
|
| [137] |
|
| [138] |
|
| [139] |
|
| [140] |
|
| [141] |
|
| [142] |
|
| [143] |
|
| [144] |
|
| [145] |
|
| [146] |
|
| [147] |
|
| [148] |
|
| [149] |
|
| [150] |
|
| [151] |
|
| [152] |
|
| [153] |
|
| [154] |
|
| [155] |
|
| [156] |
|
| [157] |
|
| [158] |
|
| [159] |
|
| [160] |
|
| [161] |
|
| [162] |
|
| [163] |
|
| [164] |
|
| [165] |
|
| [166] |
|
| [167] |
|
| [168] |
|
| [169] |
|
| [170] |
|
| [171] |
|
| [172] |
|
| [173] |
|
| [174] |
|
| [175] |
|
| [176] |
|
| [177] |
|
| [178] |
|
| [179] |
|
| [180] |
|
| [181] |
|
| [182] |
|
| [183] |
|
| [184] |
|
| [185] |
|
| [186] |
|
| [187] |
|
| [188] |
|
| [189] |
|
| [190] |
|
| [191] |
|
| [192] |
|
| [193] |
|
| [194] |
|
| [195] |
|
| [196] |
|
| [197] |
|
| [198] |
|
| [199] |
|
| [200] |
|
| [201] |
|
| [202] |
|
| [203] |
|
| [204] |
|
| [205] |
|
| [206] |
|
| [207] |
|
| [208] |
|
| [209] |
|
| [210] |
|
| [211] |
|
| [212] |
|
| [213] |
|
| [214] |
|
/
| 〈 |
|
〉 |