Abbreviation (ISO4): Prog Chem
Editor in chief: Jincai ZHAO
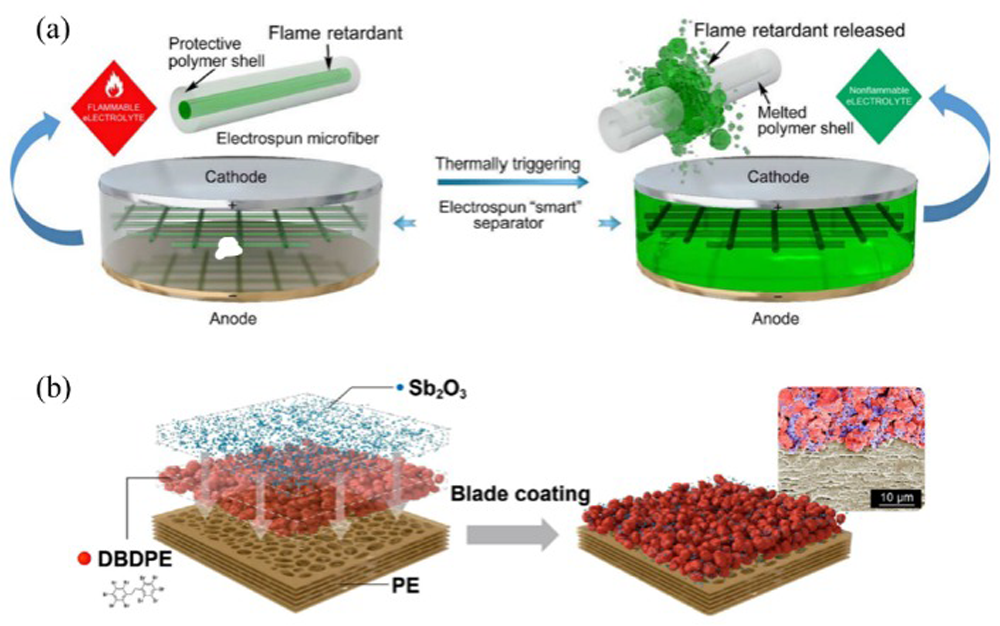
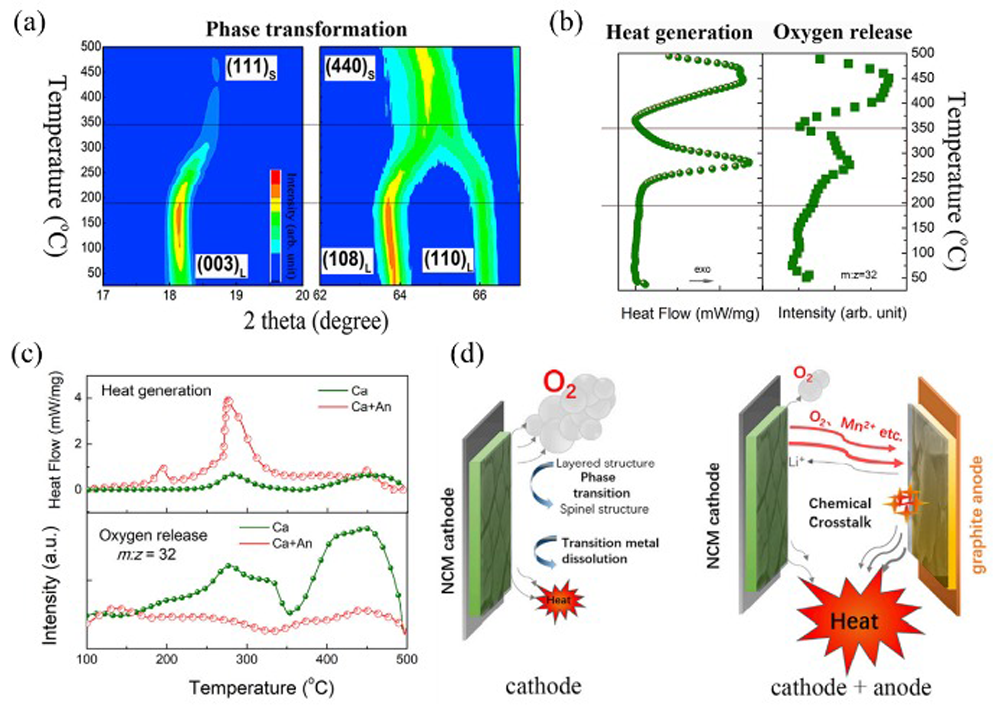 Fig.7 Thermal runaway triggered by chemical crosstalk between the cathode and anode[
Fig.7 Thermal runaway triggered by chemical crosstalk between the cathode and anode[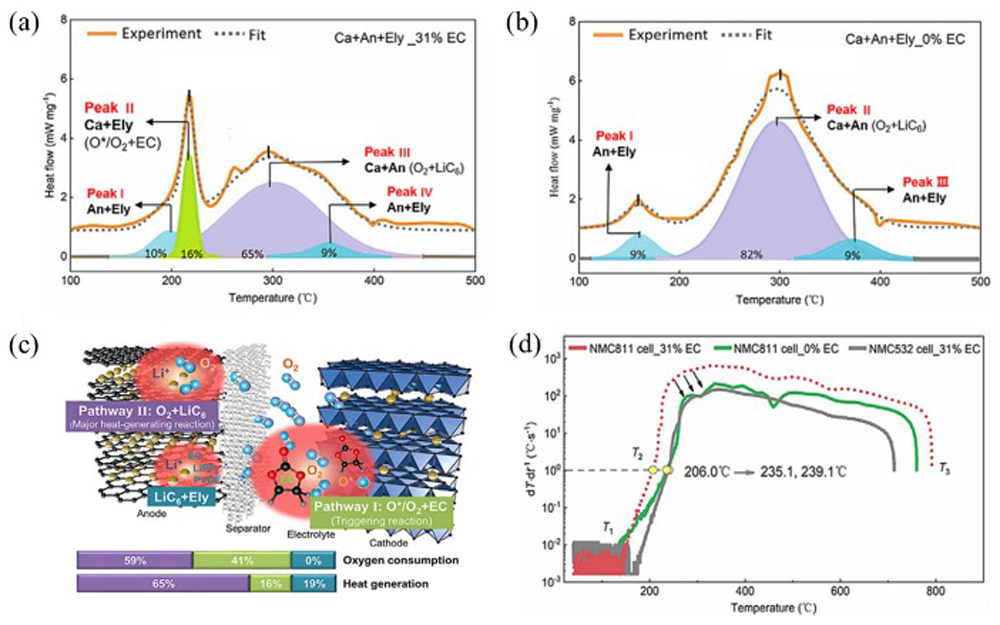 Fig.8 Two endogenous pathways of oxygen involved in thermal runaway strong exothermic reactions[
Fig.8 Two endogenous pathways of oxygen involved in thermal runaway strong exothermic reactions[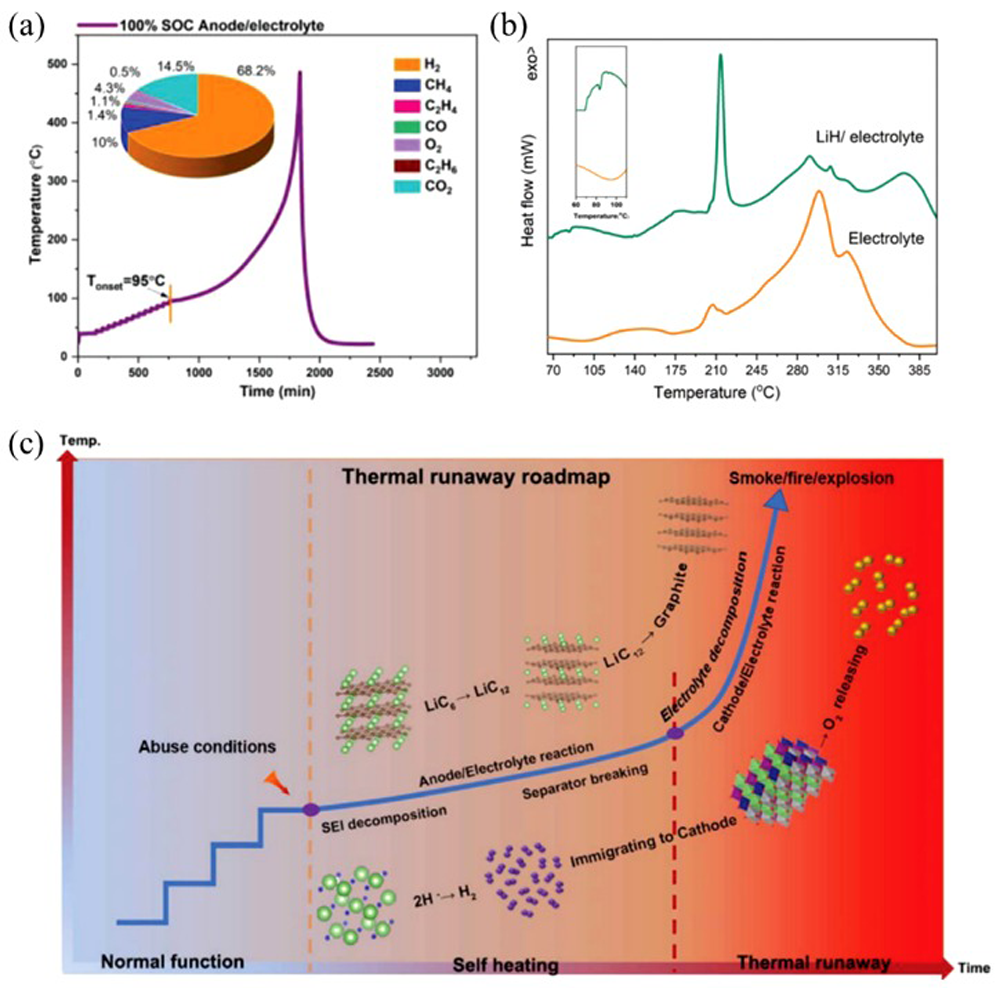 Fig.9 Thermal runaway is triggered by LiH-induced exothermic reaction at anode side and H2 migration to cathode side[
Fig.9 Thermal runaway is triggered by LiH-induced exothermic reaction at anode side and H2 migration to cathode side[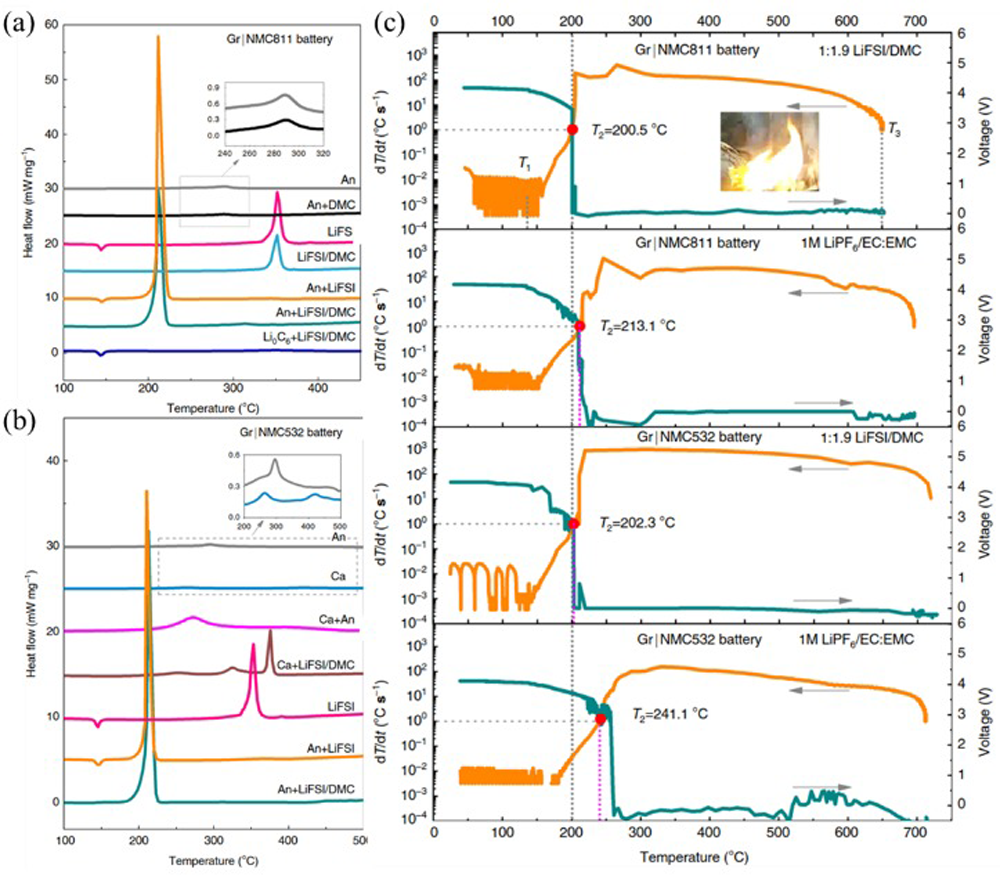 Fig.14 (a) DSC curves of components and their mixtures of NCM811/Gr battery using concentrated LiFSI/DMC electrolyte; (b) DSC curves of components and their mixtures of NCM523/Gr battery using concentrated LiFSI/DMC electrolyte; (c) comparison of thermal runaway features of NCM/Gr batteries with concentrated LiFSI/DMC and conventional 1 M LiPF6/EC:EMC electrolyte[
Fig.14 (a) DSC curves of components and their mixtures of NCM811/Gr battery using concentrated LiFSI/DMC electrolyte; (b) DSC curves of components and their mixtures of NCM523/Gr battery using concentrated LiFSI/DMC electrolyte; (c) comparison of thermal runaway features of NCM/Gr batteries with concentrated LiFSI/DMC and conventional 1 M LiPF6/EC:EMC electrolyte[